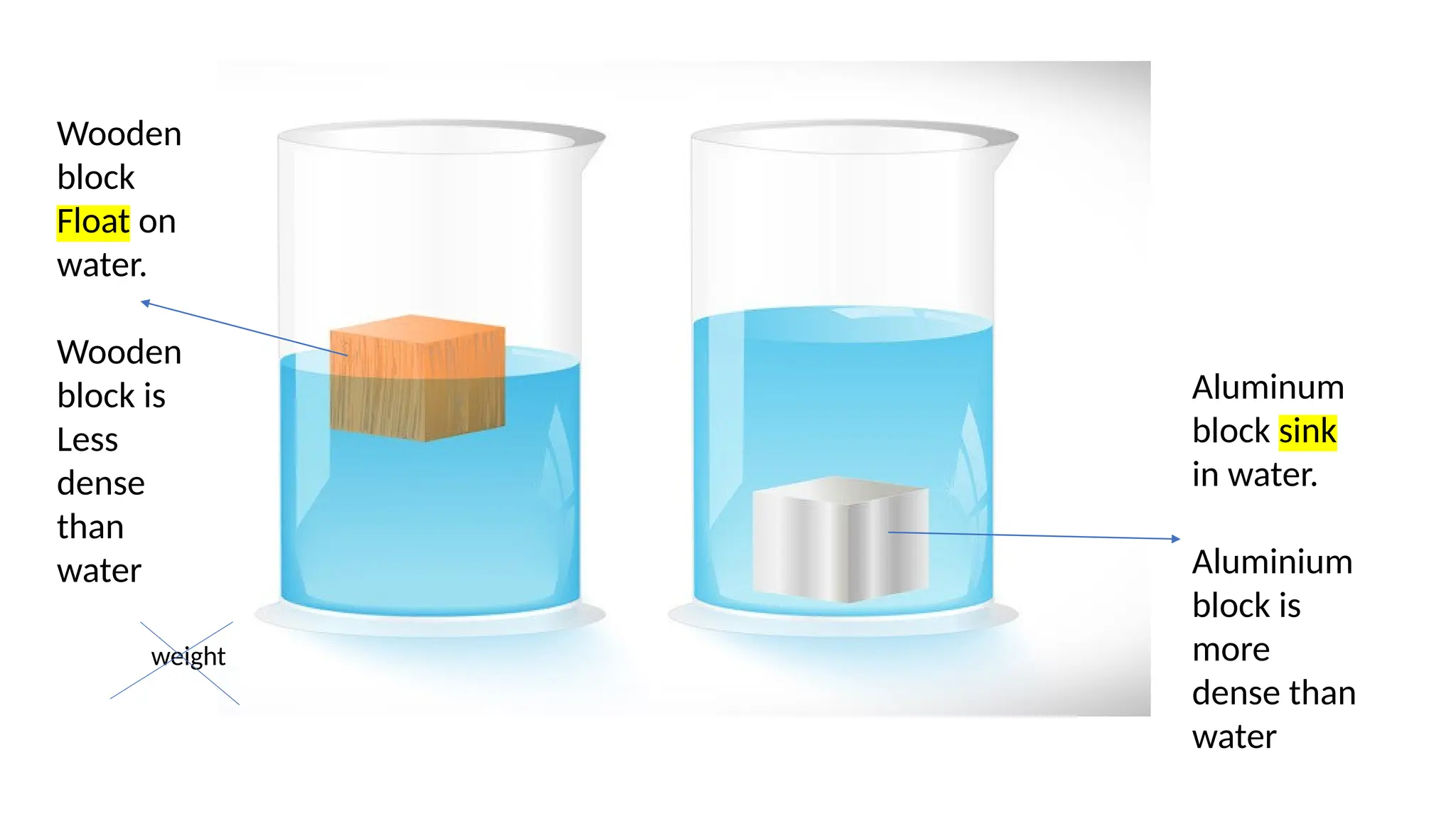
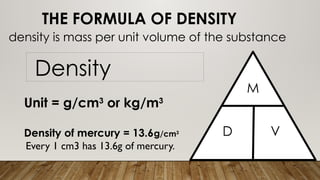
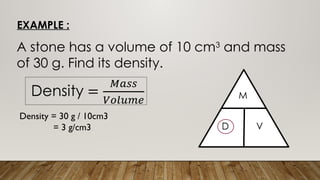
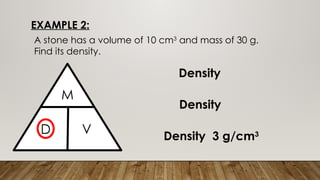
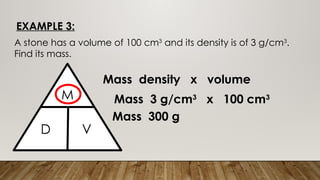
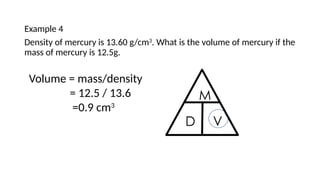
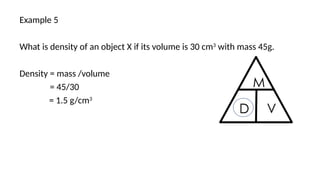
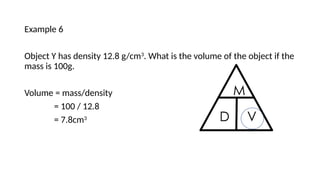
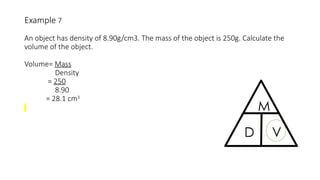
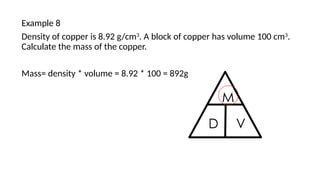
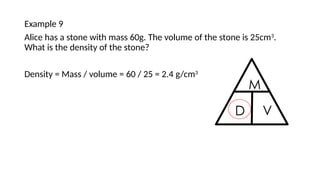
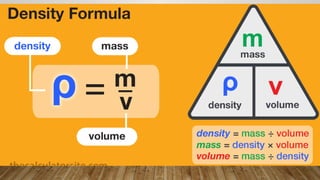
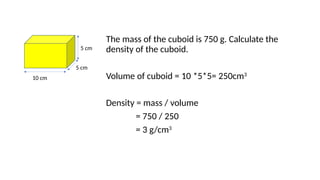
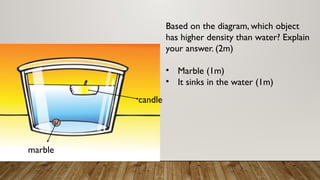
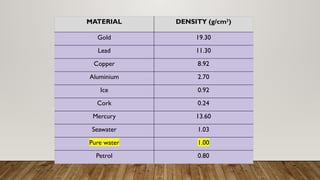
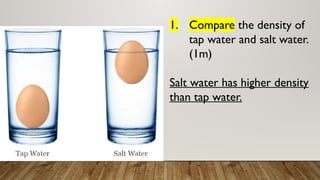
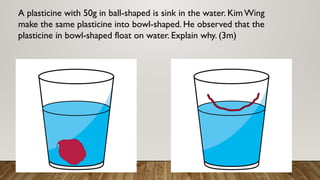
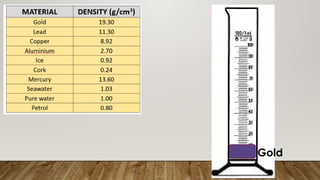
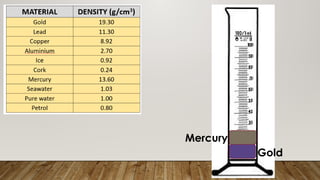
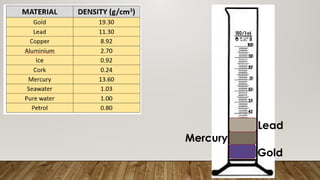
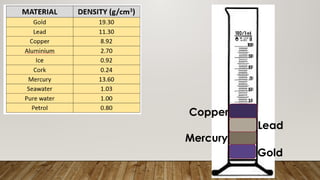
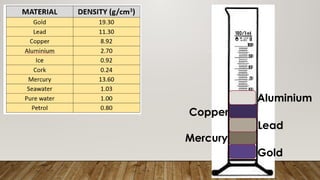
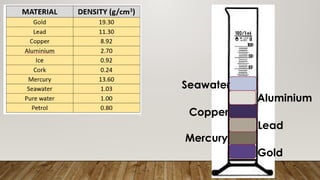
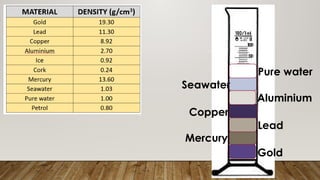
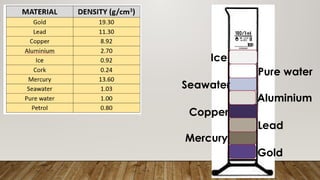
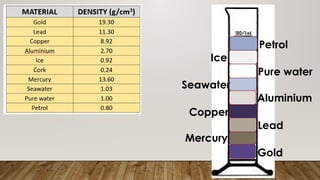
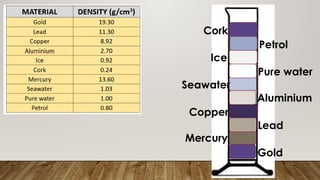
This document discusses the concept of density, detailing how different materials float or sink in water based on their density. It provides various examples and calculations for determining density and volume, using specific formulas and methods like water displacement. Additionally, it explores practical applications of density in everyday life and provides comparisons between the densities of various materials.