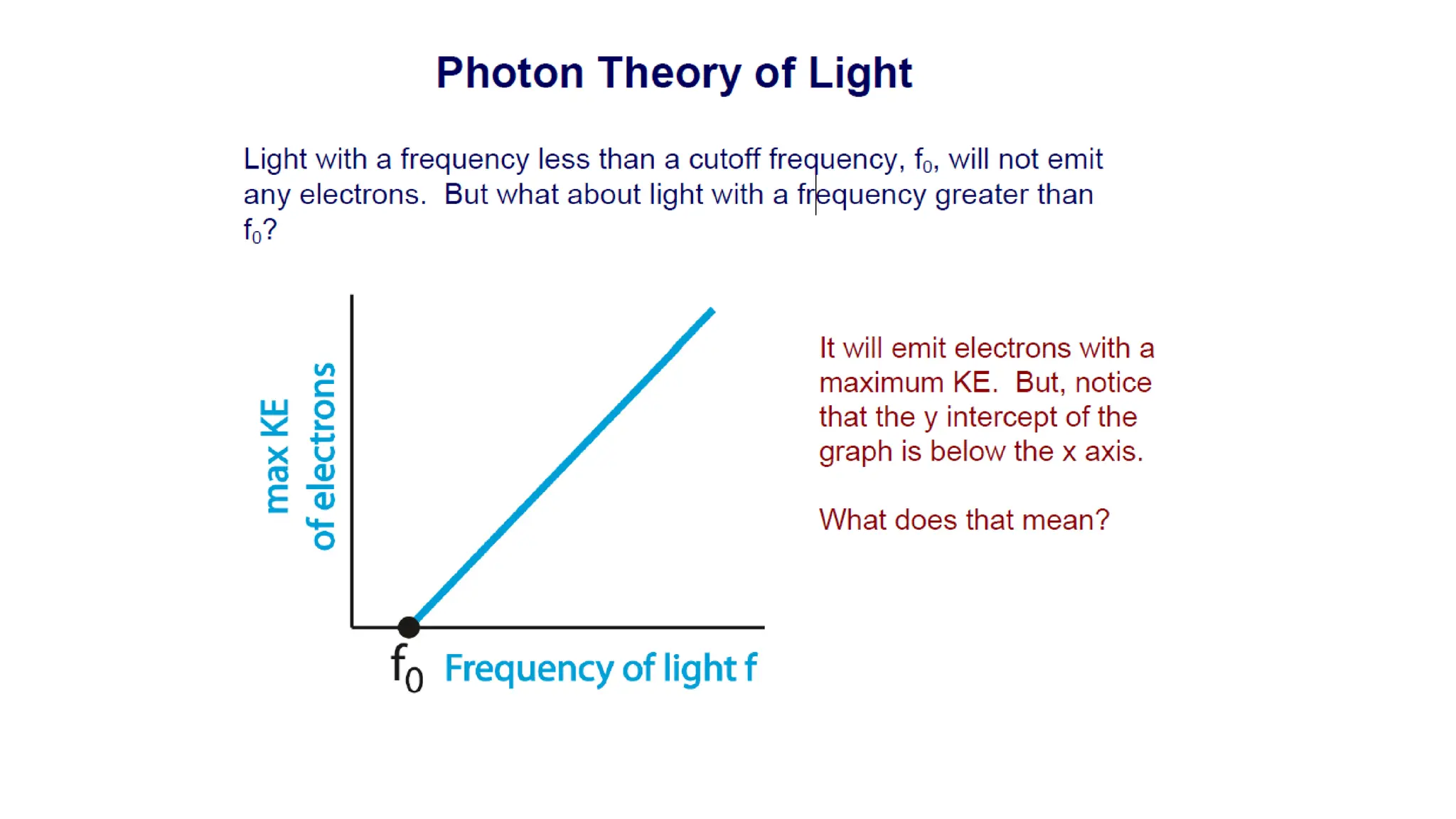
The document discusses key elements of modern physics, including concepts like blackbody radiation, the photoelectric effect, and the Bohr model of the atom. It highlights the limitations of Bohr's model and the importance of energy levels in atoms, while also detailing various applications of lasers and phenomena like Fraunhofer lines. Additionally, it poses practice questions to test understanding of the discussed concepts.