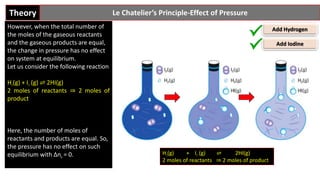
Le Chatelier's principle states that if a stress is applied to a system at equilibrium, it will shift in a way to reduce the effect of the stress. For reactions with gaseous components, an increase in pressure favors the side with fewer moles of gas, while a decrease in pressure favors the side with more moles of gas. However, if the total moles of gas are equal on both sides of the reaction, as in the reaction H2(g) + I2(g) ⇌ 2HI(g), a change in pressure will not affect the equilibrium position.