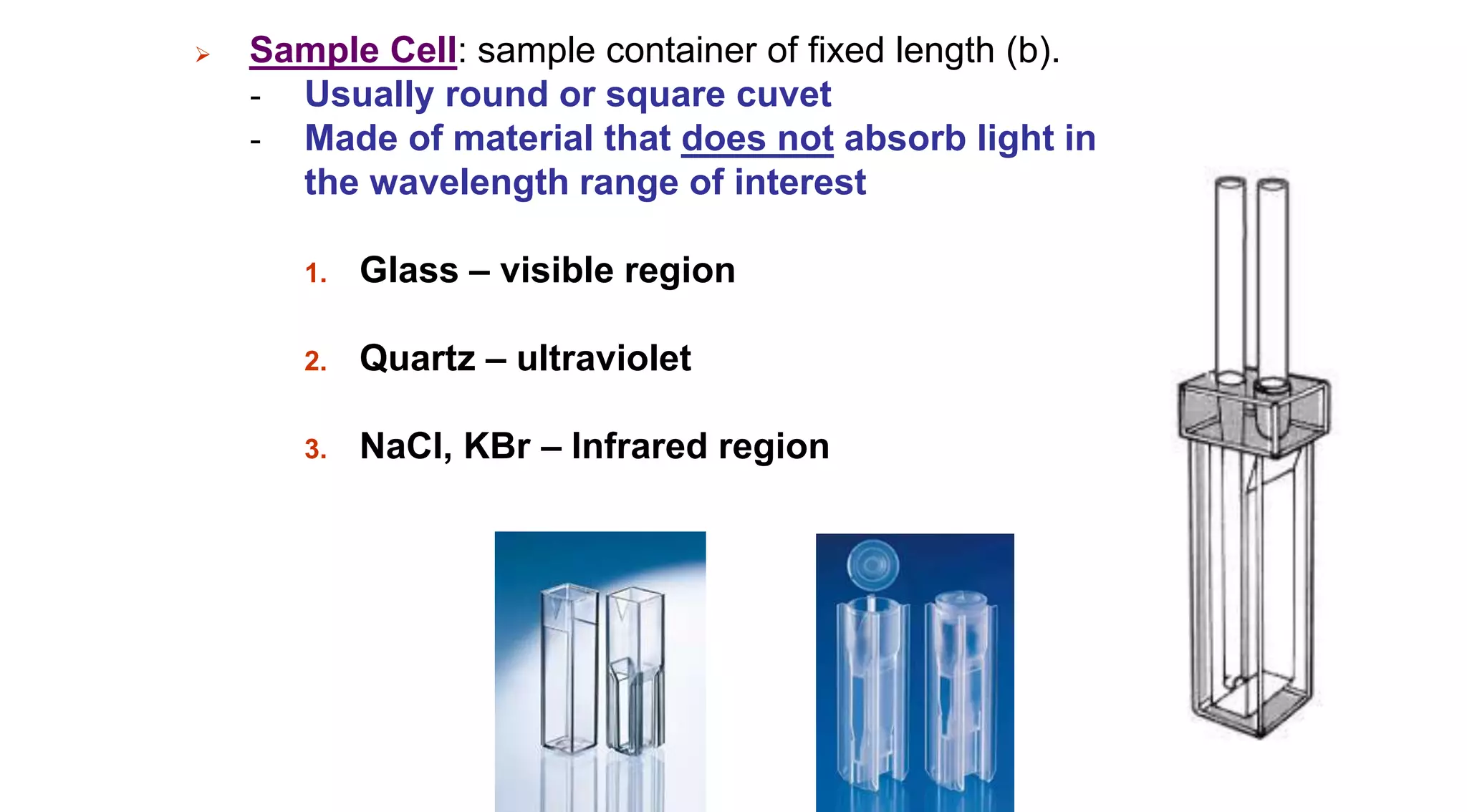
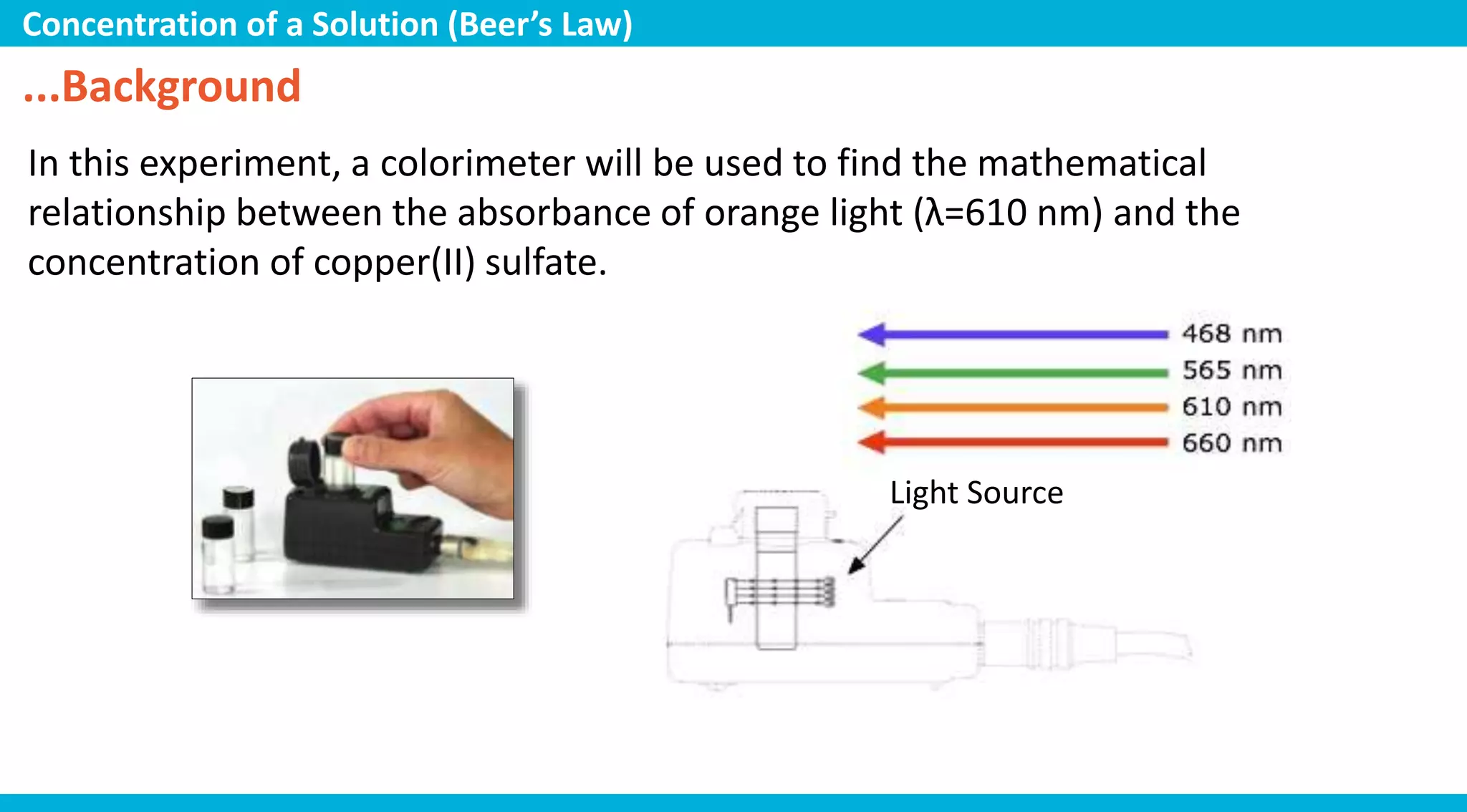
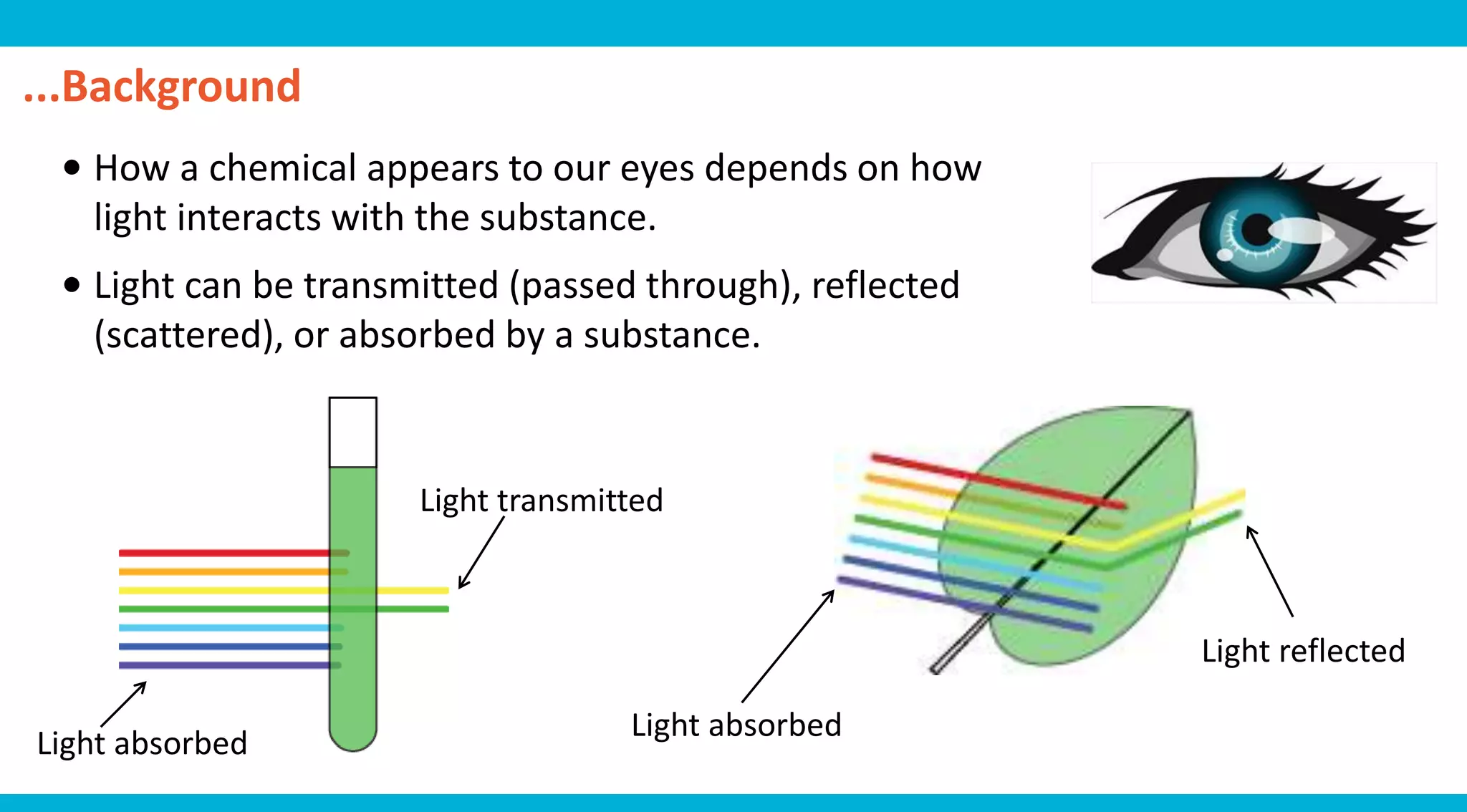
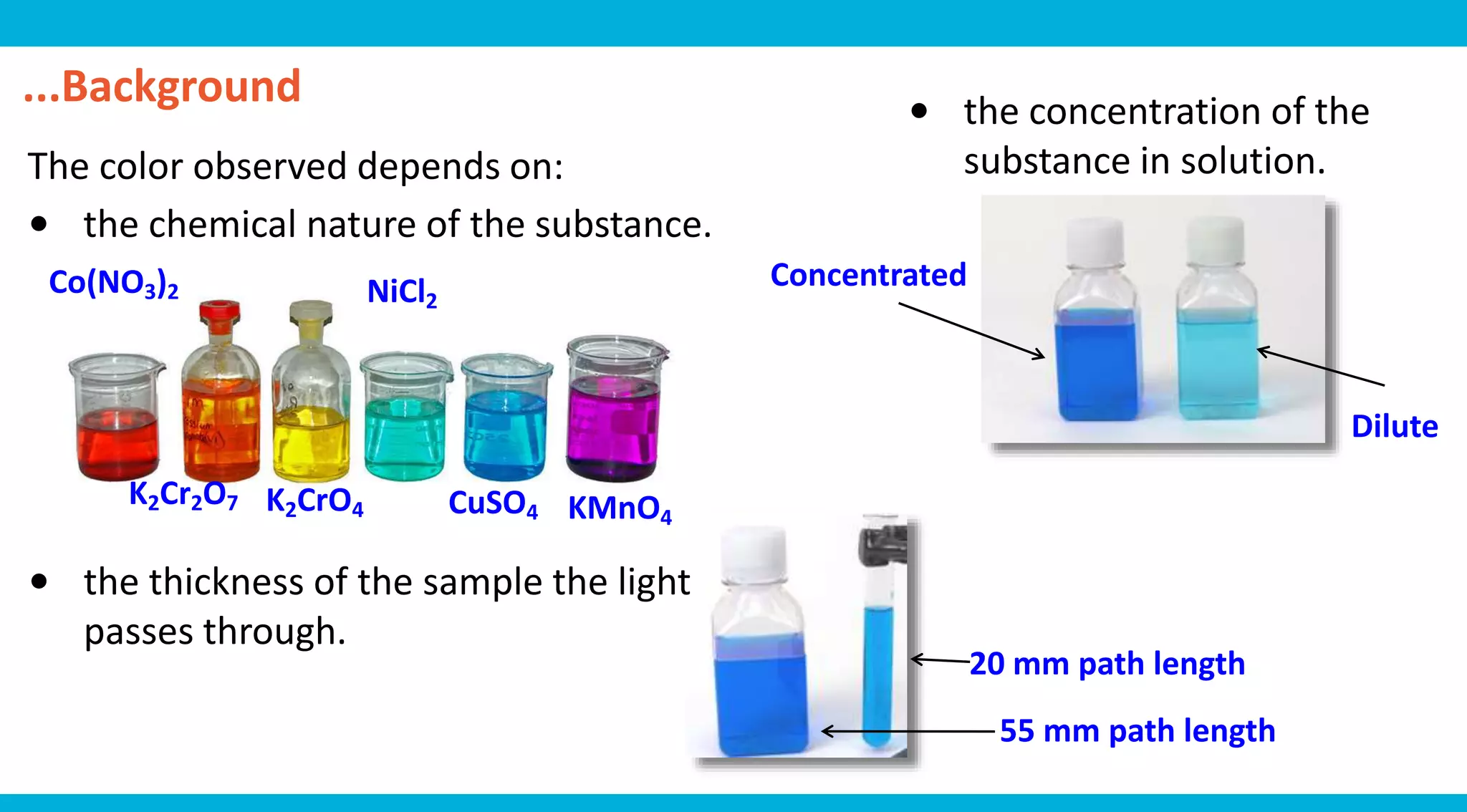
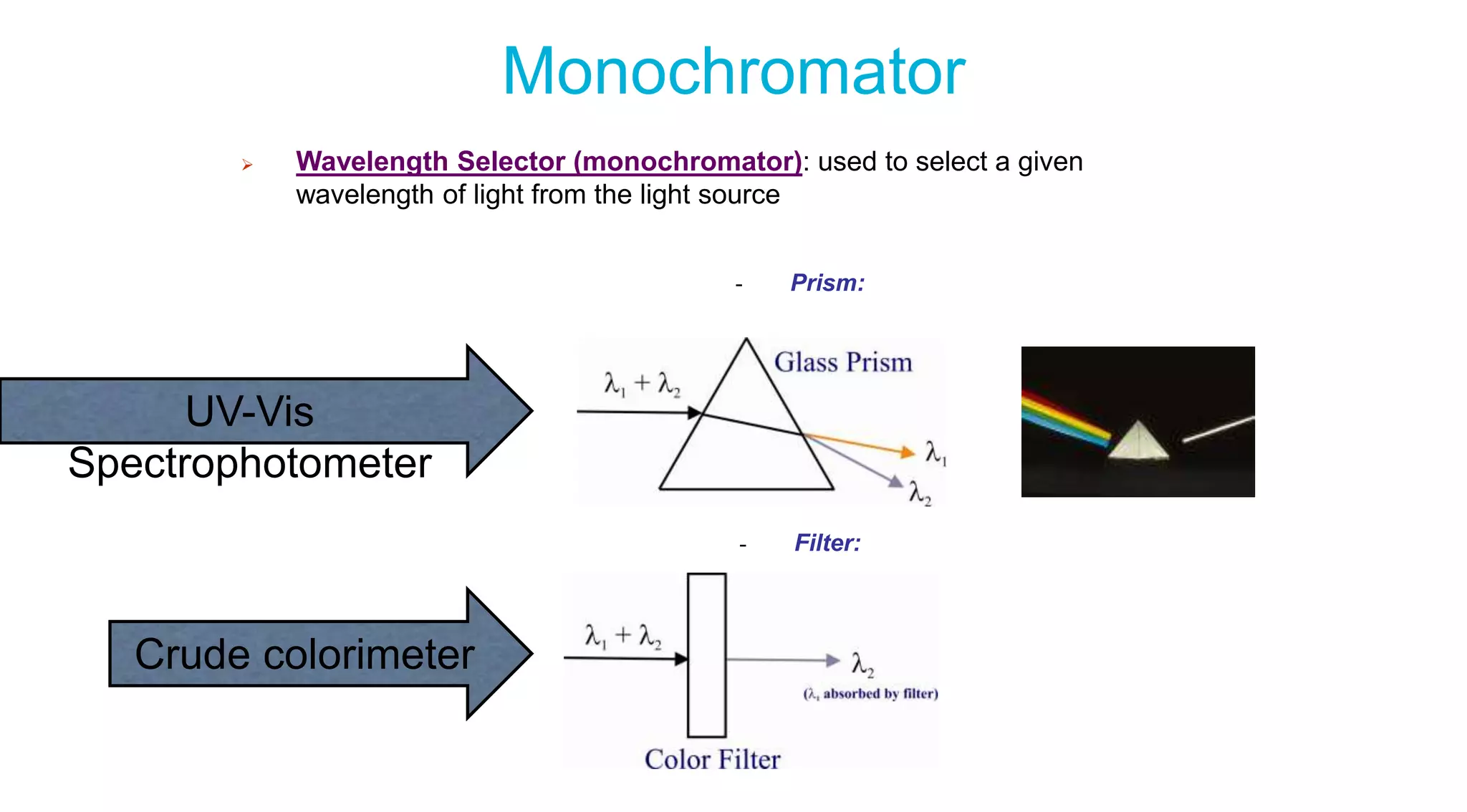
This document discusses colorimetry and UV-visible spectroscopy techniques for identifying and quantifying ions in aqueous solutions. It explains that these techniques analyze the wavelengths of light absorbed by chemical substances to determine their identity and concentration. Beer's law states that absorbance is directly proportional to analyte concentration, molar absorptivity, and path length. The steps of a procedure using a colorimeter to determine the concentration of copper(II) sulfate solutions are outlined.





![Beer’s Law
The relative amount of a certain wavelength of light absorbed (A) that passes through a sample is
dependent on:
- distance the light must pass through the sample (cell path length - b)
- amount of absorbing chemicals in the sample (analyte concentration – c)
- ability of the sample to absorb light (molar absorptivity - e)
Increasing [Fe2+]
Absorbance is directly proportional to concentration of Fe+2](https://image.slidesharecdn.com/l5usingcolorimeteruvvisspectrometrysimplified-230726230904-45663a98/75/L5-Using-Colorimeter-UV-Vis-Spectrometry-simplified-pptx-10-2048.jpg)