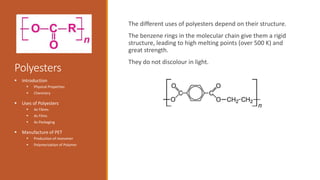
The document discusses polyamides and polyesters, detailing their formation, types, and applications. It covers the history of nylon, the discovery of Kevlar, and the development of Nomex, along with their chemical structures and uses. Additionally, it explains polyester fibers, their manufacturing process, and various applications in clothing and packaging.


![Polyamides
History
Nylon => Developed in the 1930’s at DuPont by Wallace
Carothers and his team of researchers.
Nylon is formed by the condensation reaction of two
components:
Diamine (a compound containing two amino [NH2]
groups—e.g., hexamethylenediamine)
Dicarboxylic acid (containing two carboxyl [CO−OH]
groups—e.g., adipic acid)
Or may be formed by the self-condensation of an amino acid
or an amino-acid derivative.
Introduction
Nylon
Chemistry
Applications
Kevlar
Chemistry
Applications
Nomex
Chemistry
Applications
Nylon](https://image.slidesharecdn.com/kim510ecarbonyladd-elimination3-180505084833/85/Kim510-e-carbonyl-add-elimination-3-3-320.jpg)