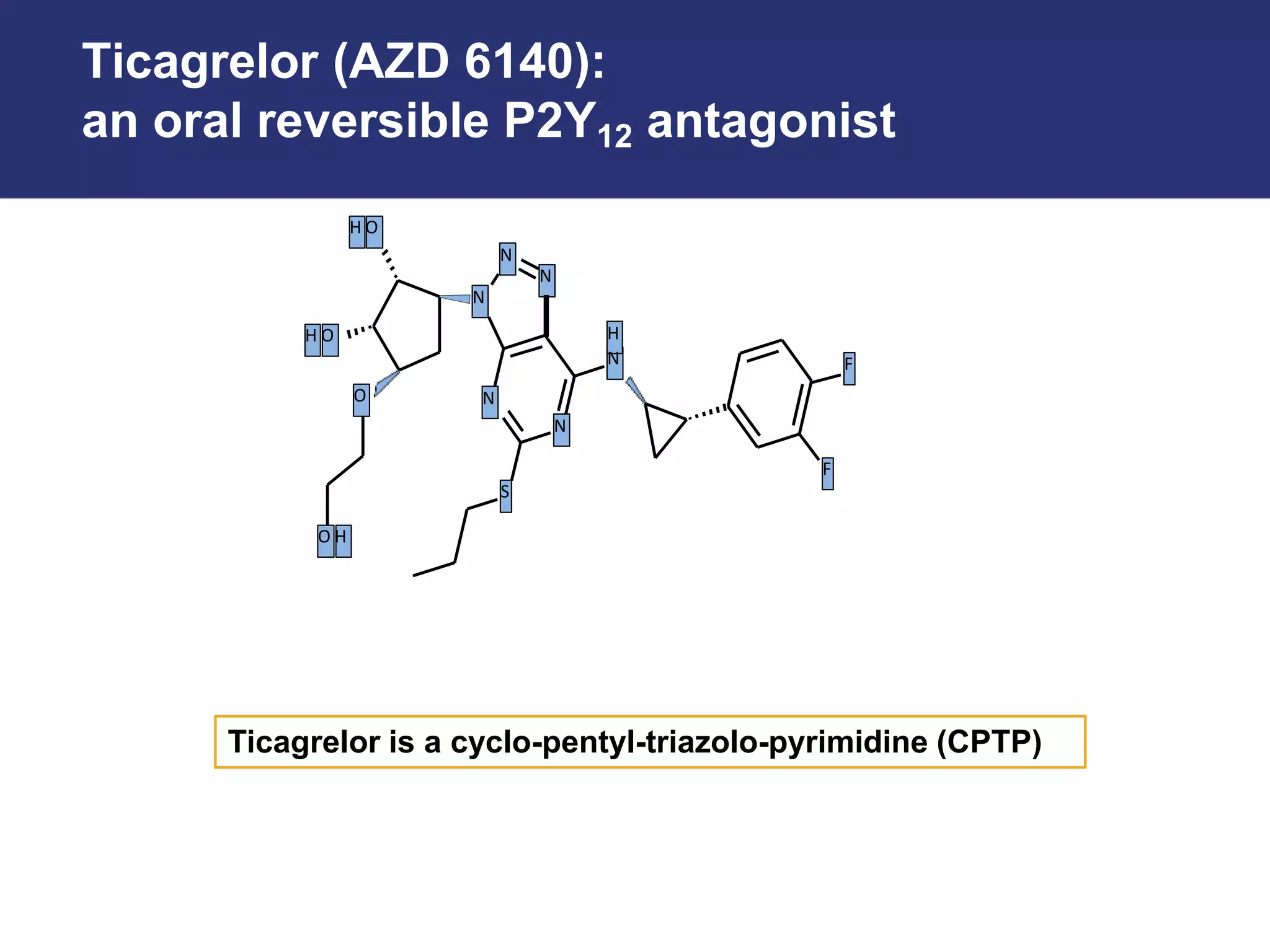
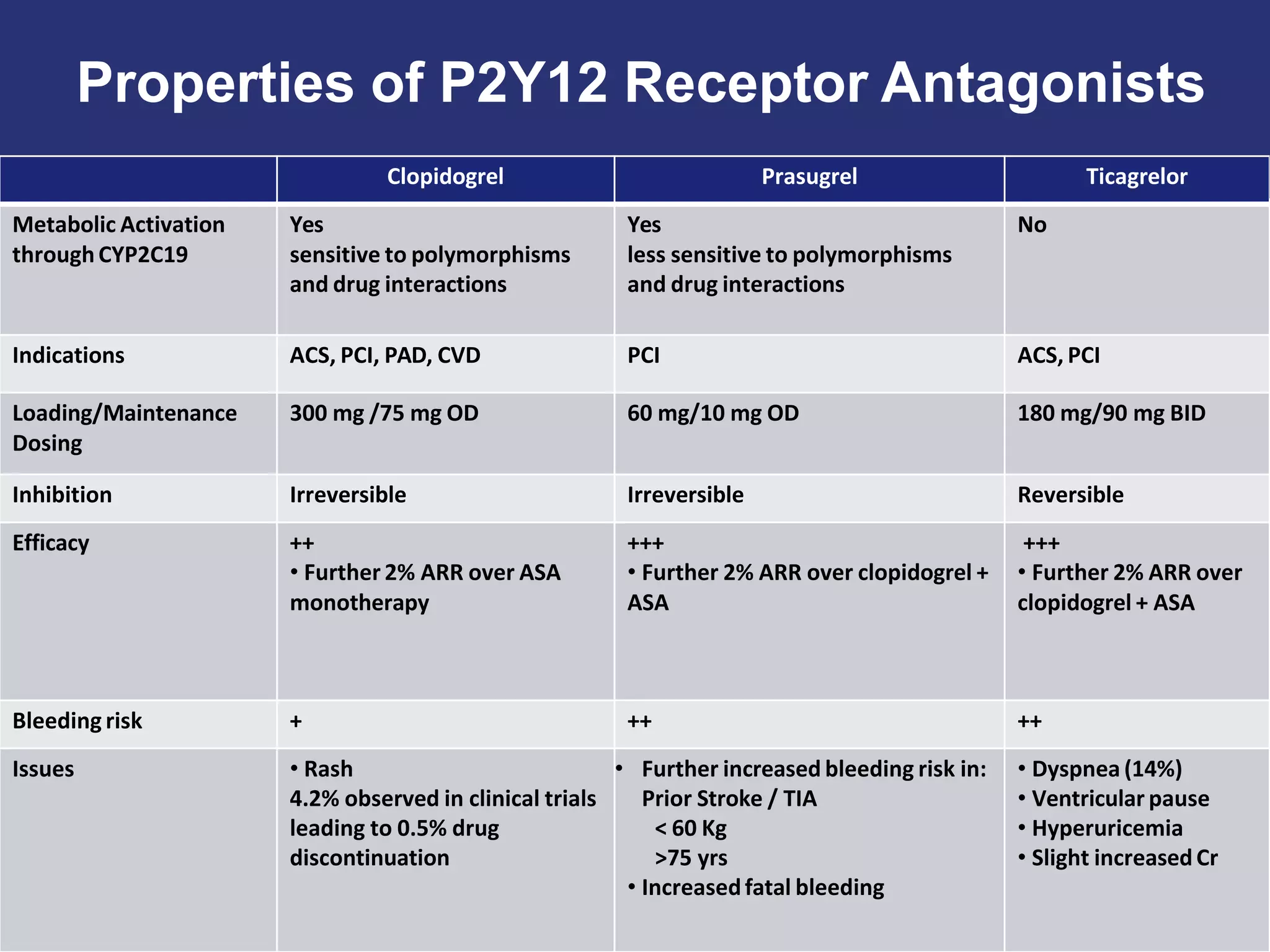
Ticagrelor is a reversible P2Y12 platelet inhibitor that was developed as an alternative to clopidogrel for dual antiplatelet therapy following acute coronary syndromes or percutaneous coronary interventions.
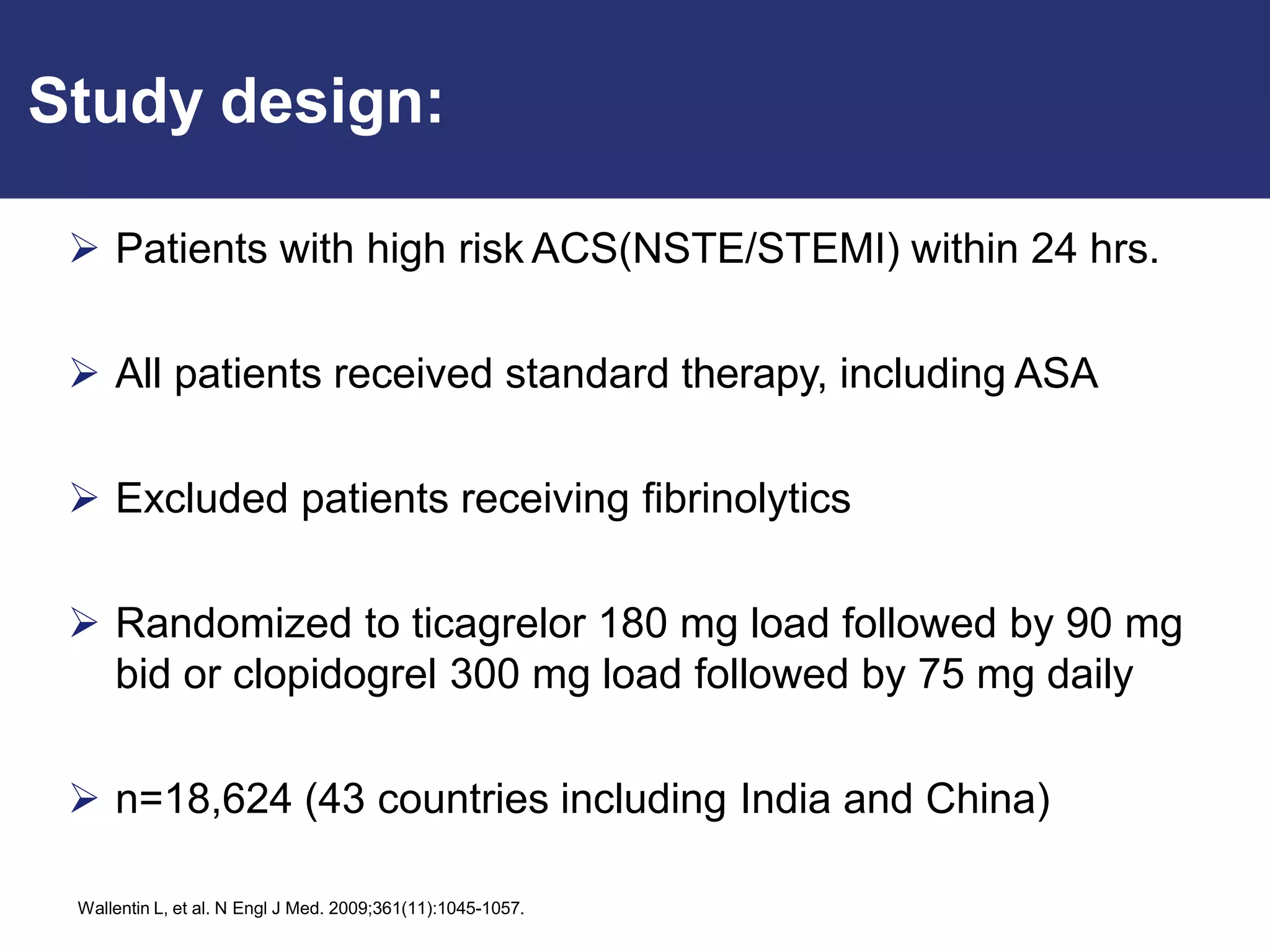
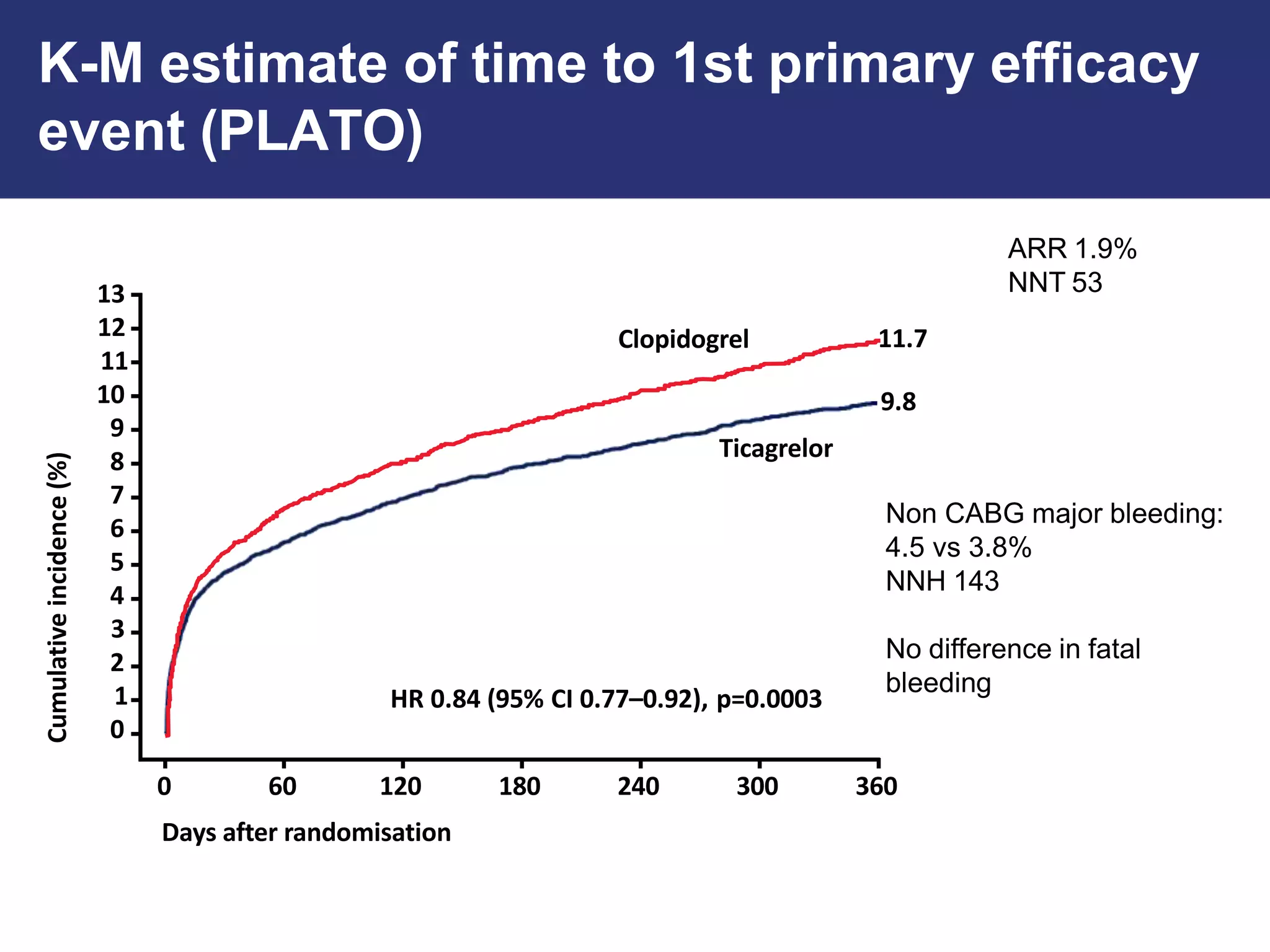
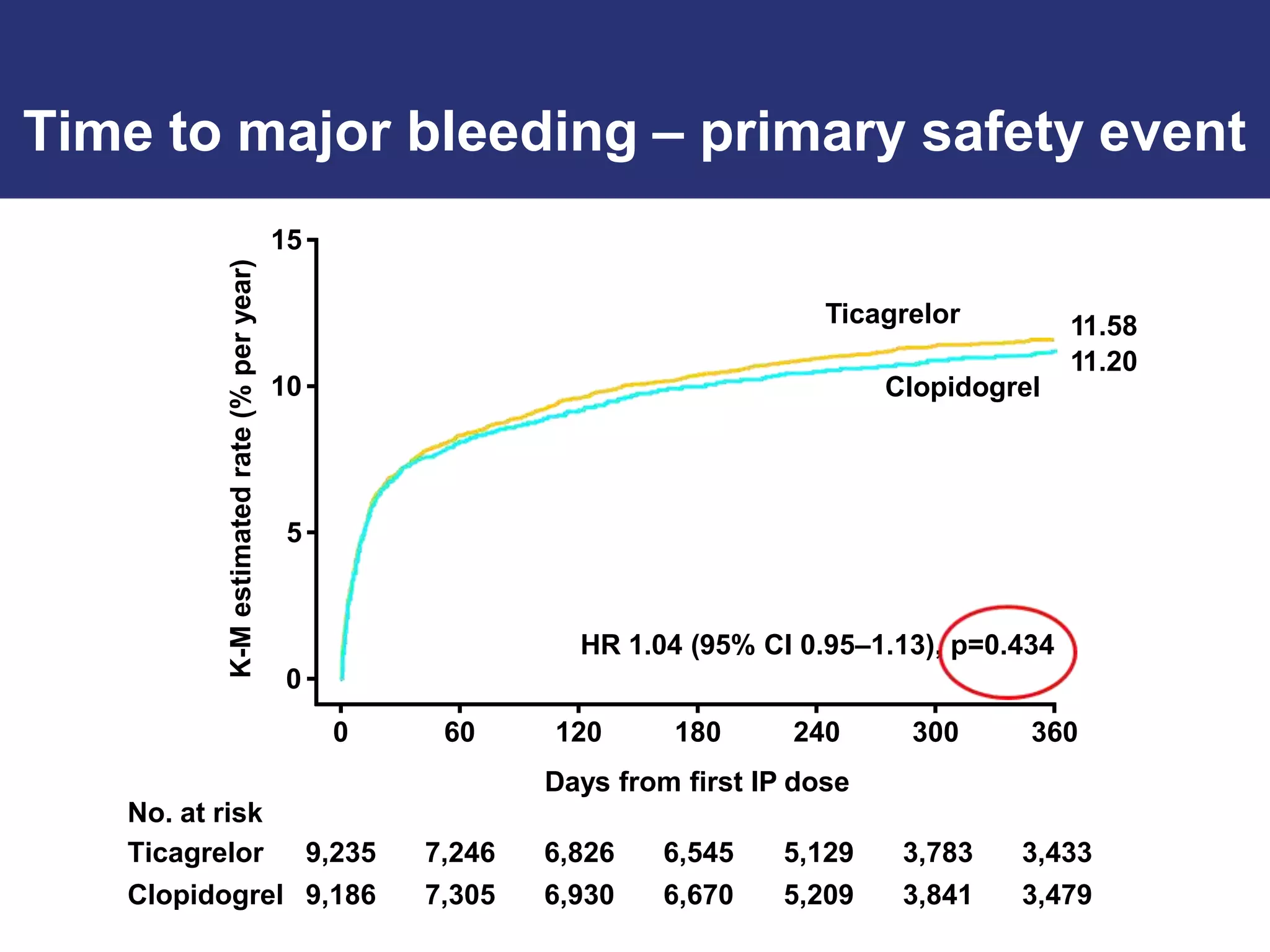
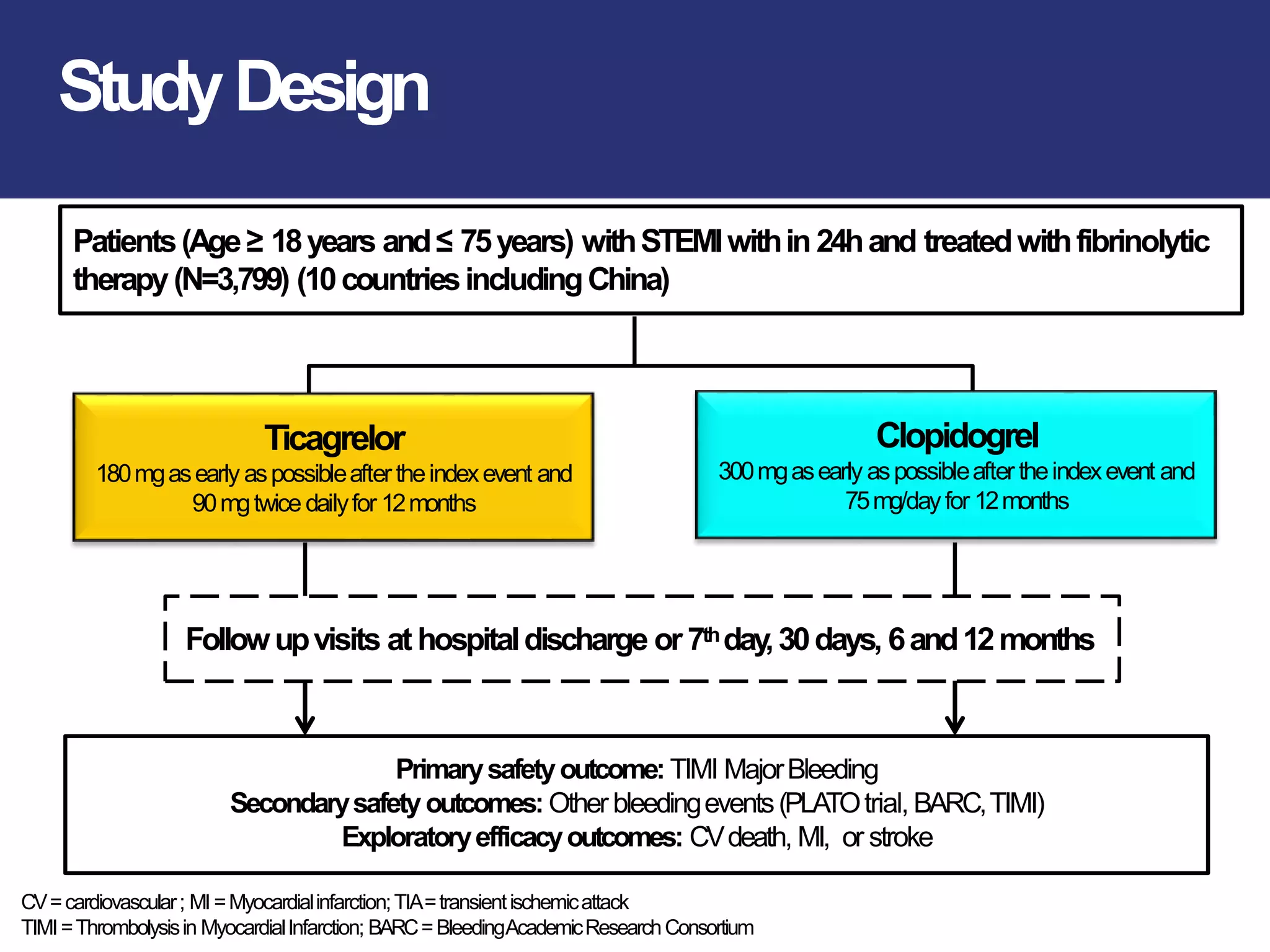
The PLATO trial found that ticagrelor was more effective than clopidogrel at reducing the primary endpoint of cardiovascular death, myocardial infarction, and stroke in patients with acute coronary syndrome, with no significant difference in major bleeding risks. However, ticagrelor was associated with higher rates of dyspnea and asymptomatic ventricular pauses.
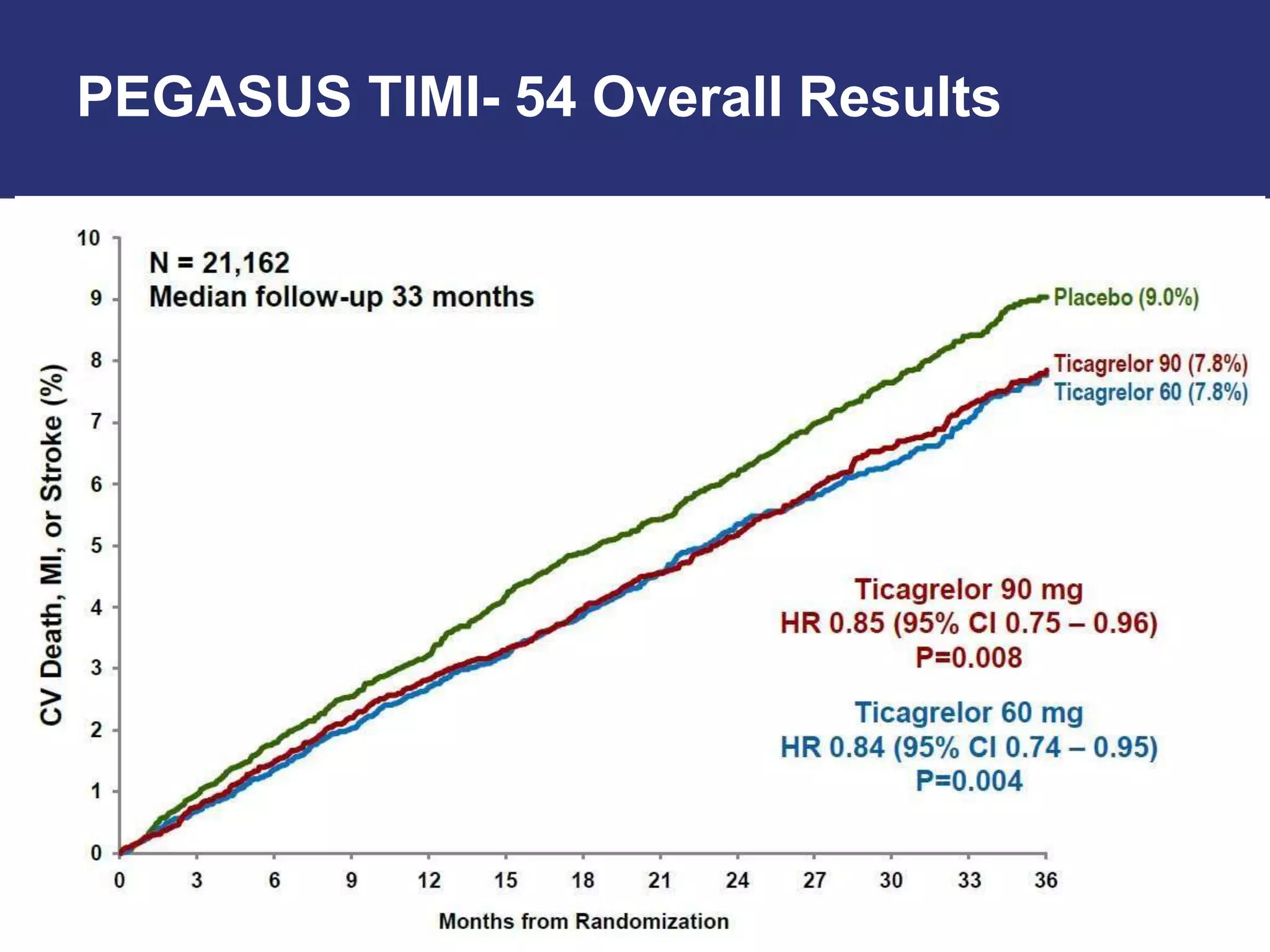
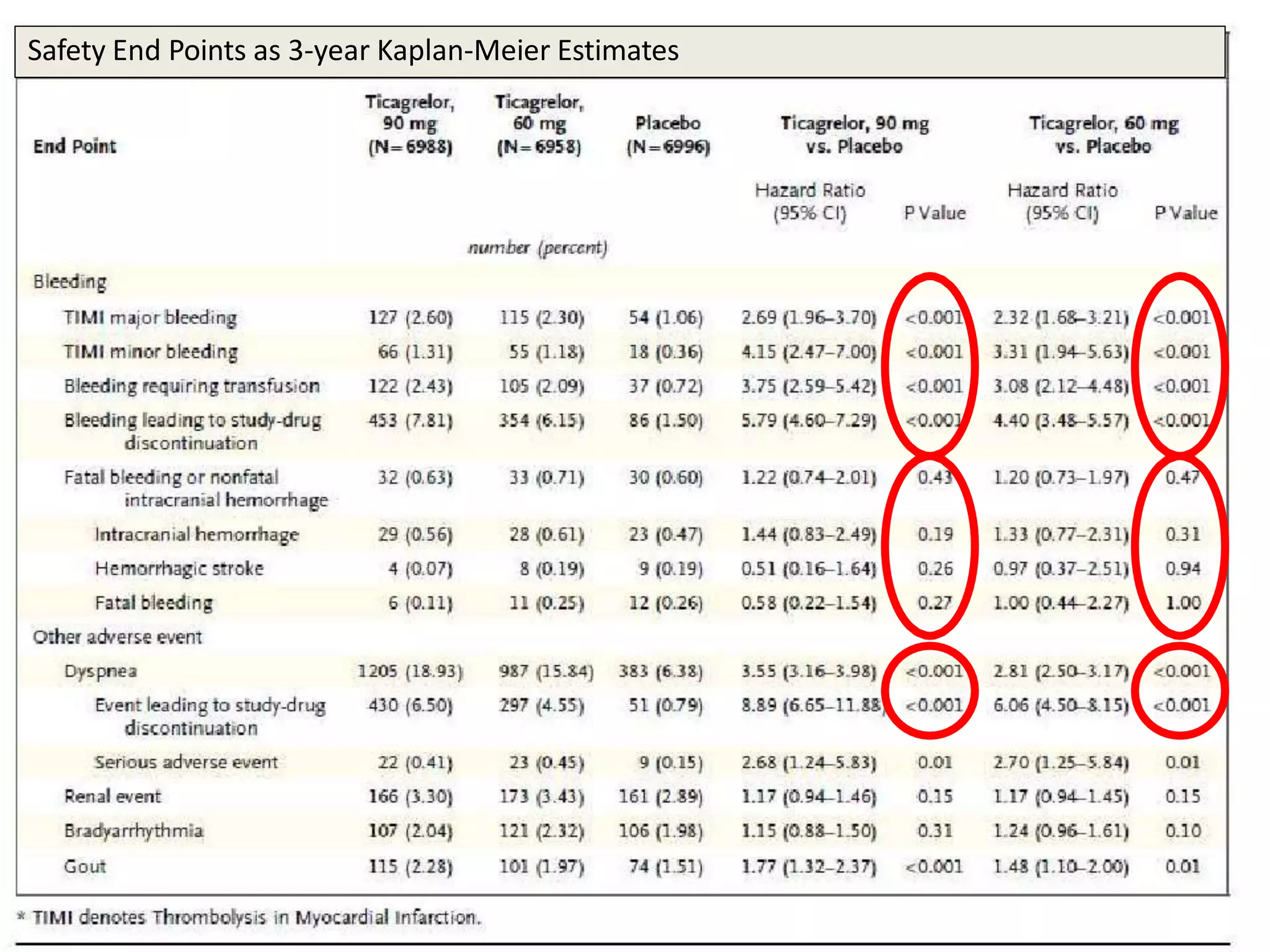
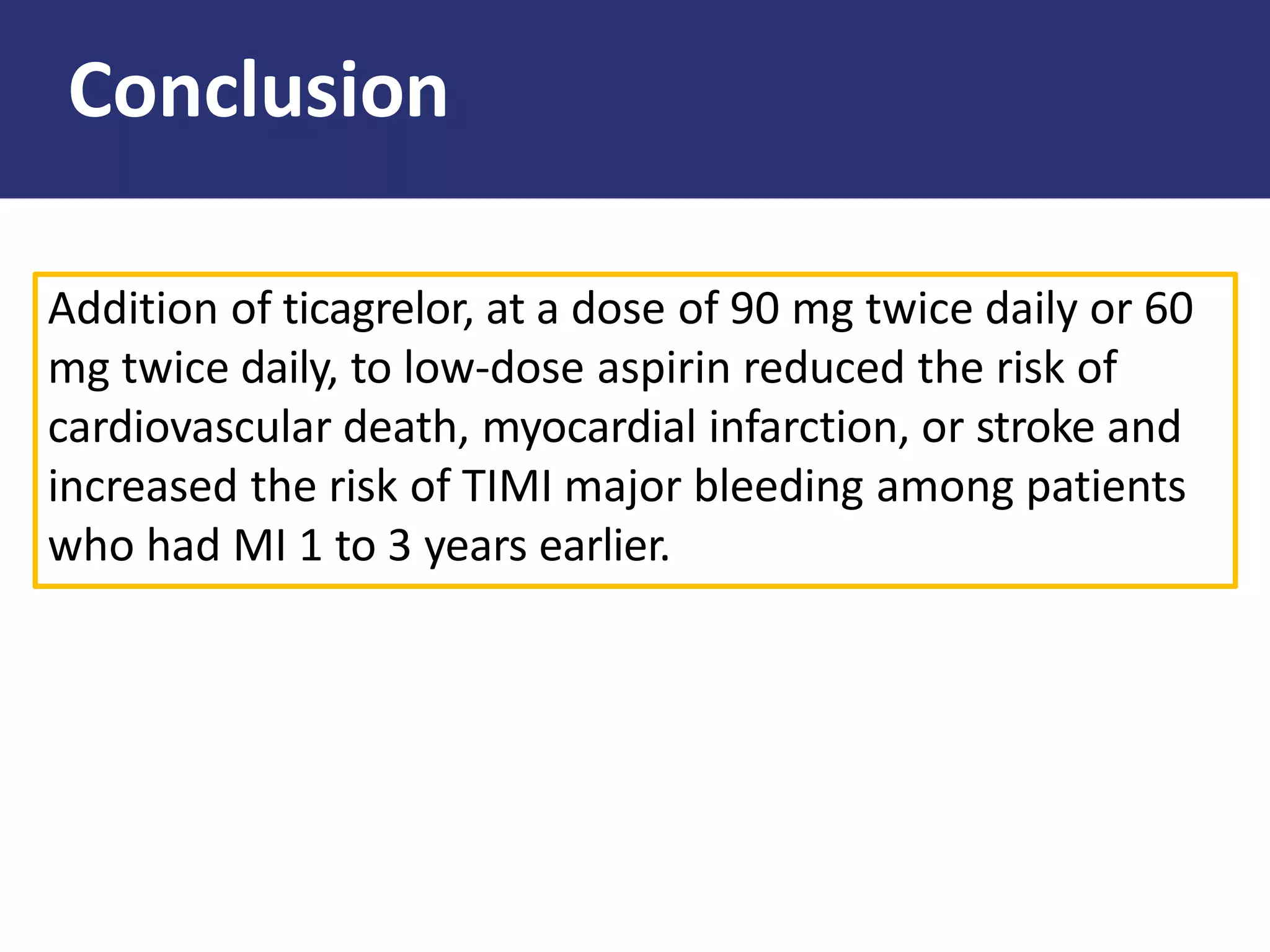
The PEGASUS trial showed that long-term use of ticagrelor on a background of aspirin reduced the risk of cardiovascular events in patients