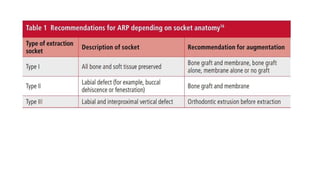
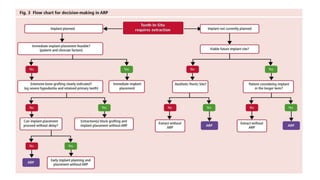
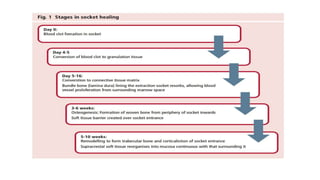
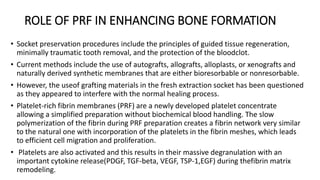
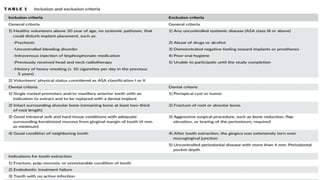
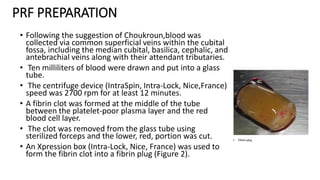
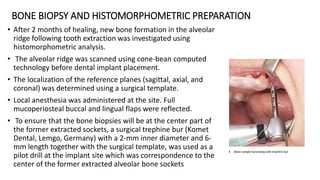
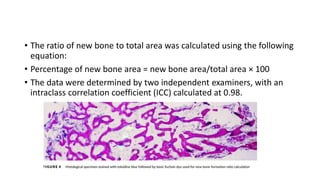
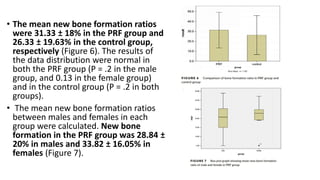
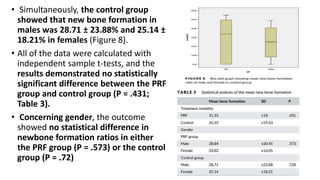
This randomized controlled trial compared new bone formation in alveolar bone sockets following tooth extraction when treated with platelet-rich fibrin (PRF) versus normal wound healing. Thirty-three patients underwent minimally traumatic tooth extractions, with 18 sockets treated with PRF placed in the socket and 15 sockets left to heal naturally. Bone specimens were taken 2 months post-extraction and analyzed histomorphometrically. The study found a higher percentage of new bone formation with PRF treatment compared to the control, but the difference was not statistically significant. The use of PRF in alveolar socket preservation did not enhance new bone formation compared to normal wound healing.