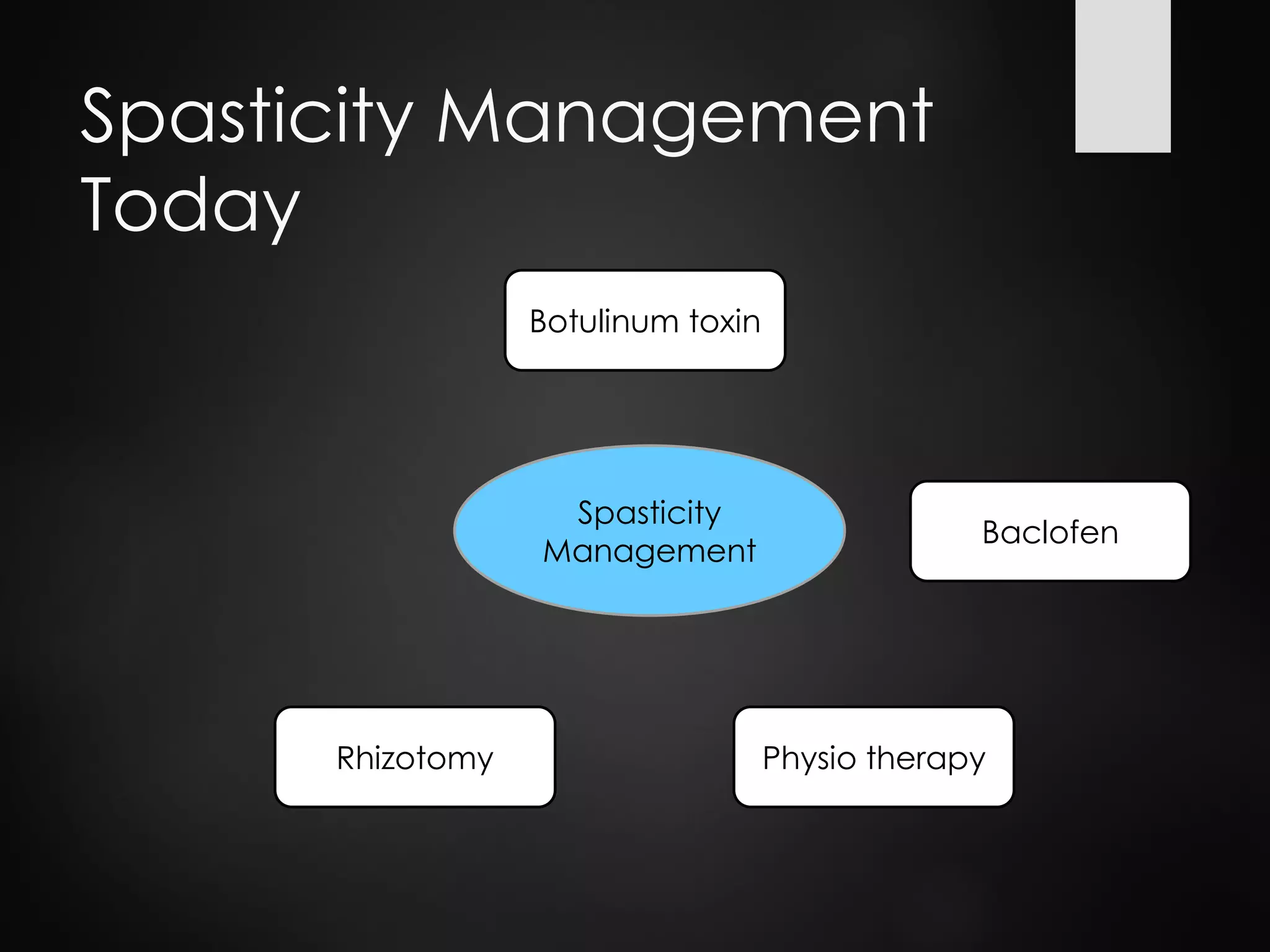
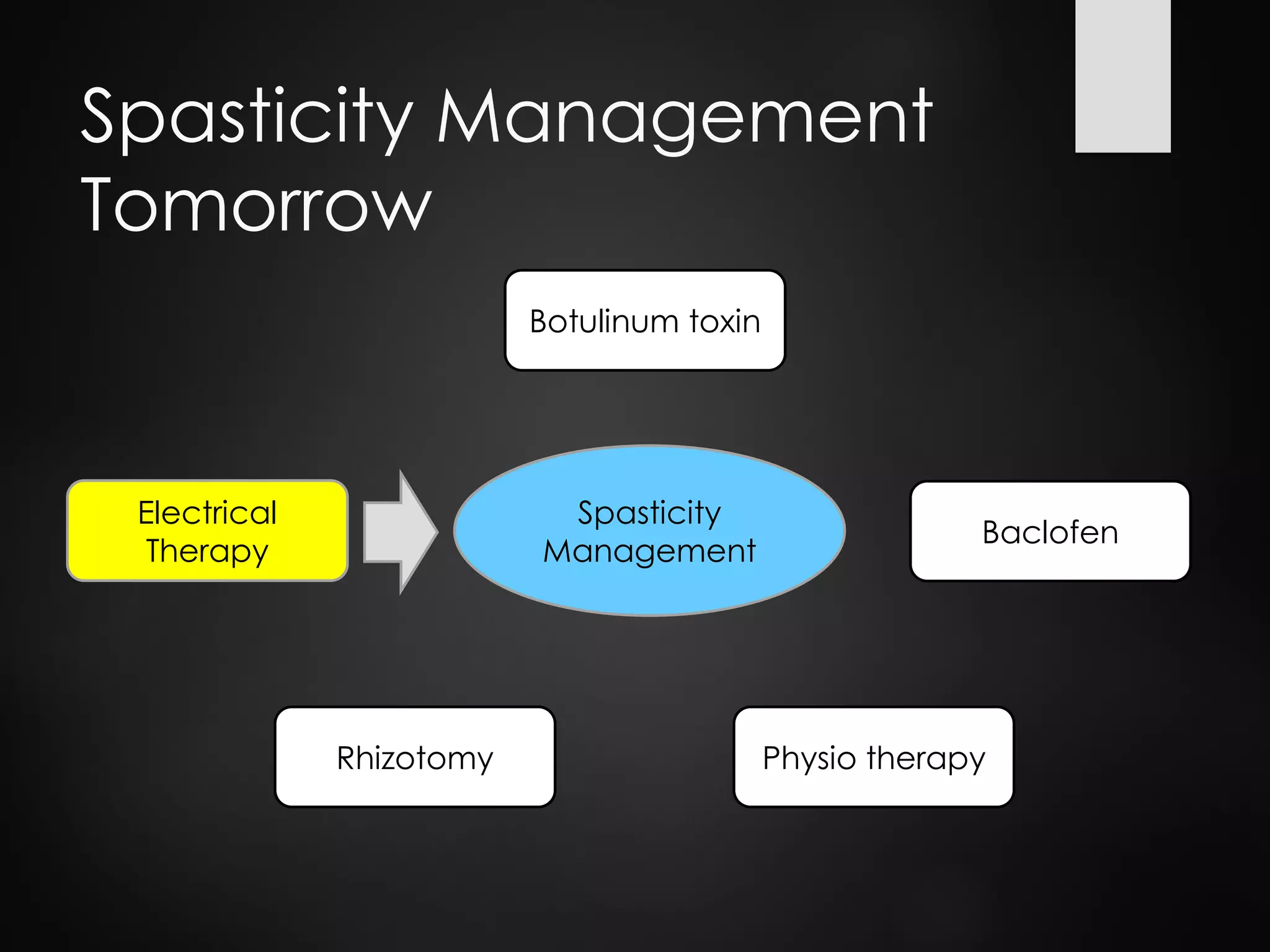
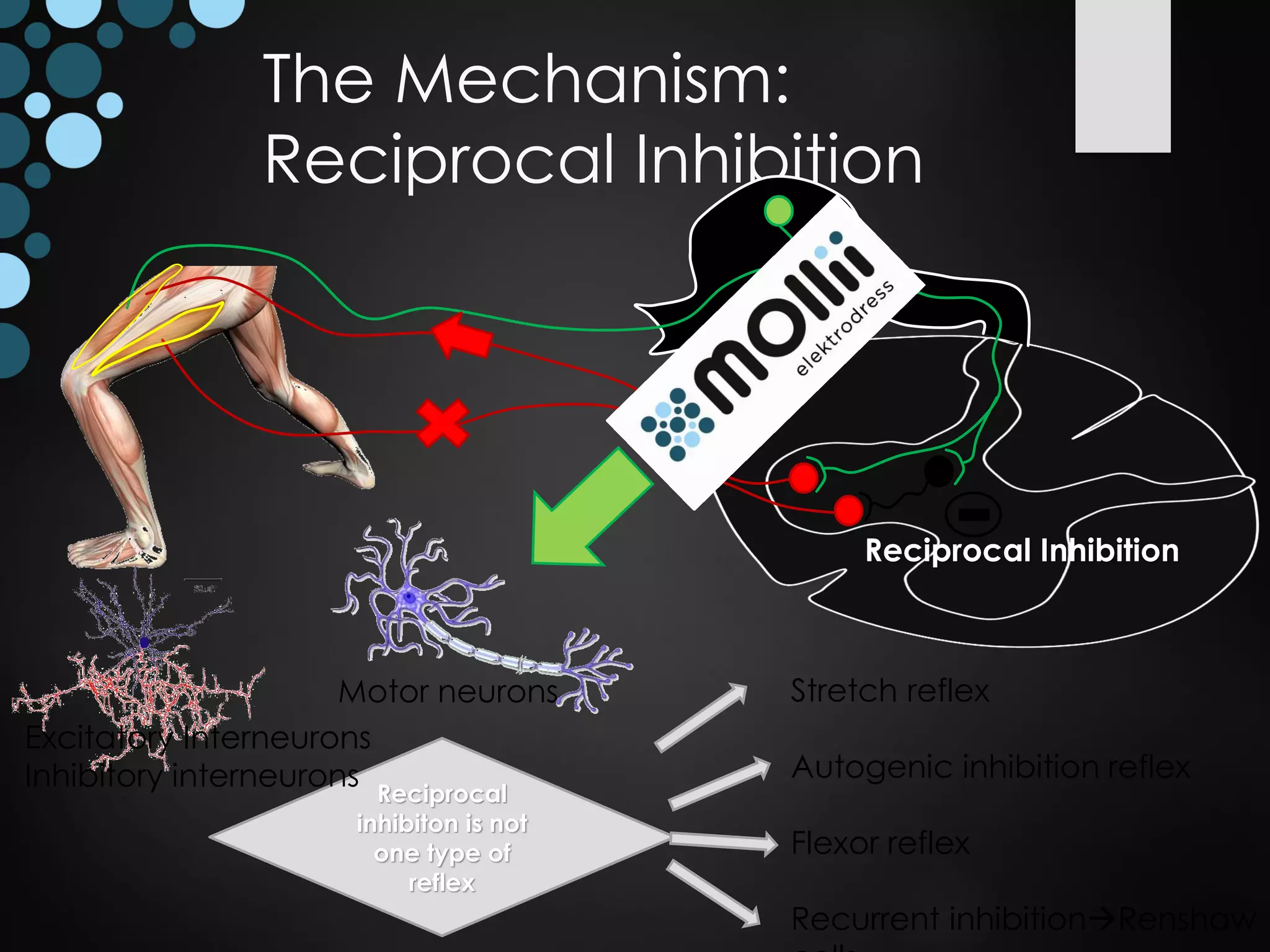
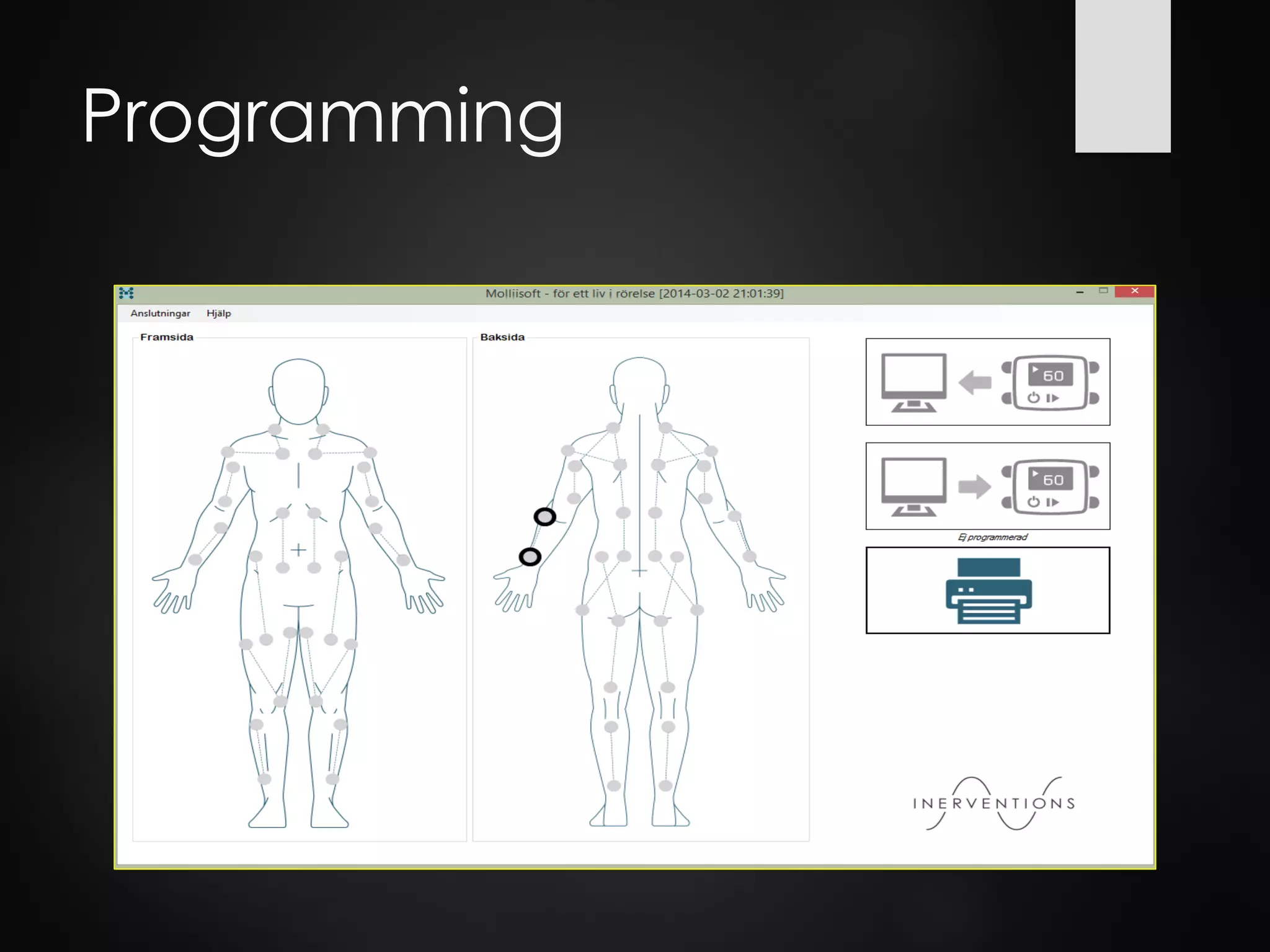
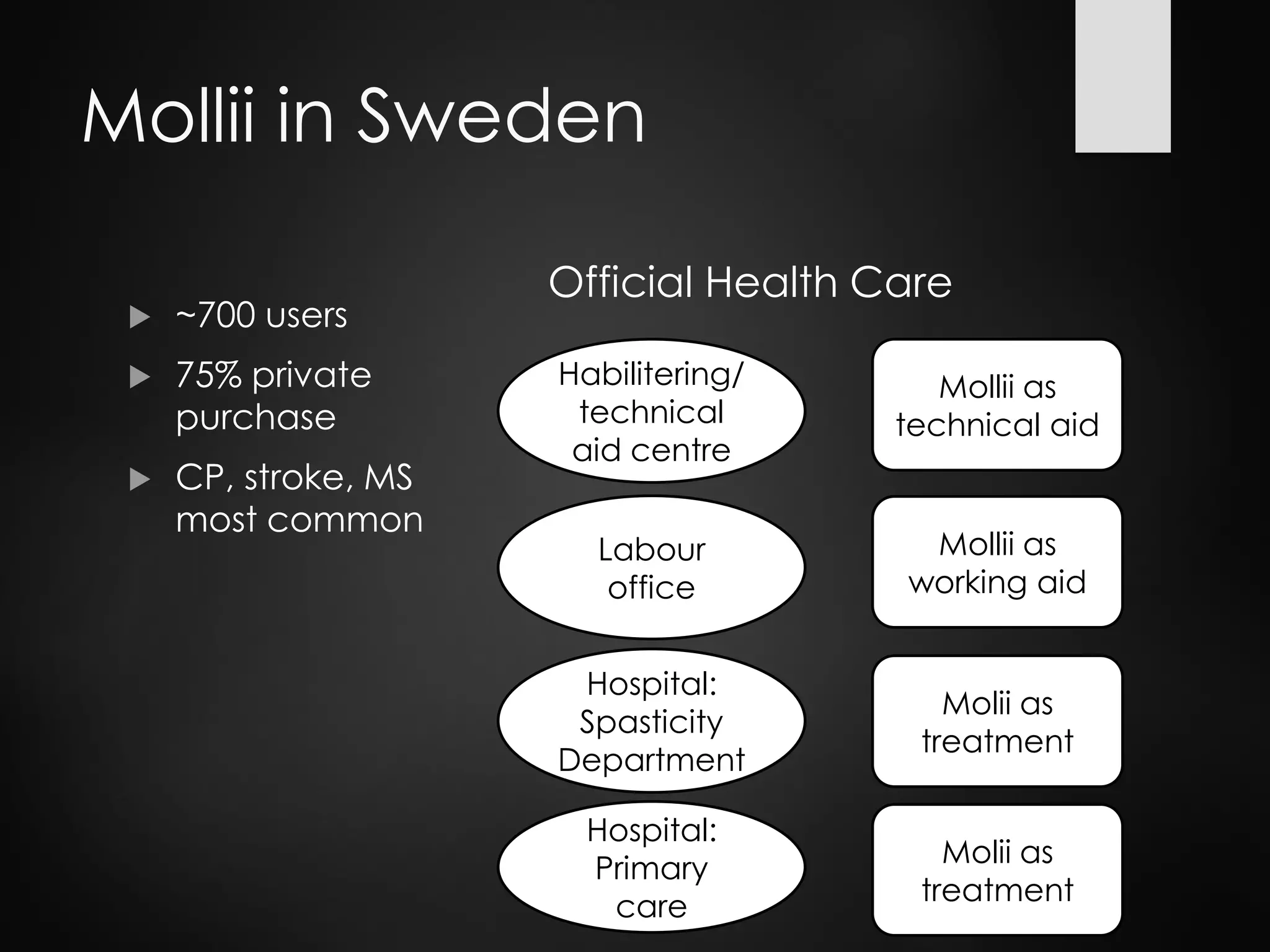
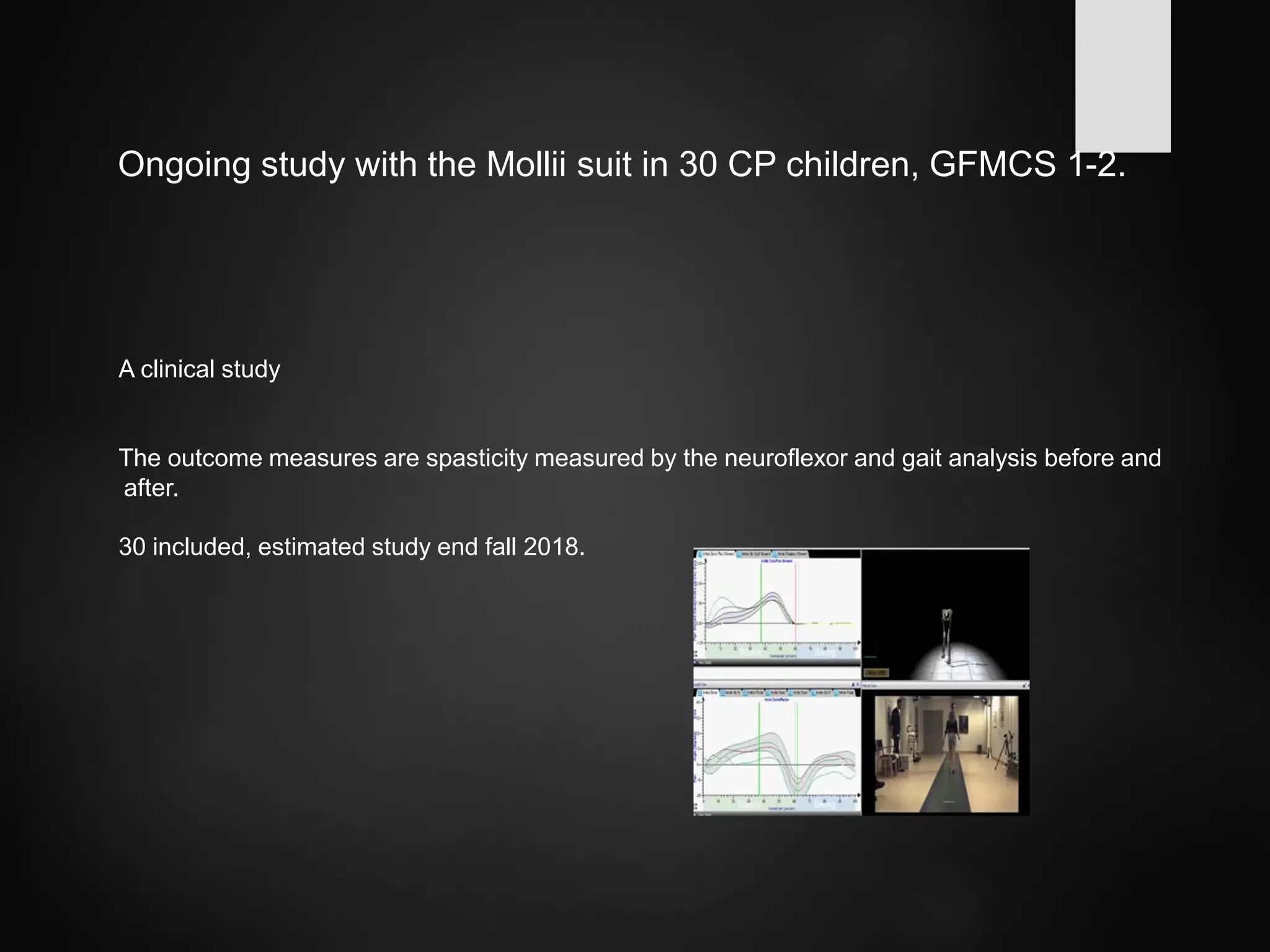
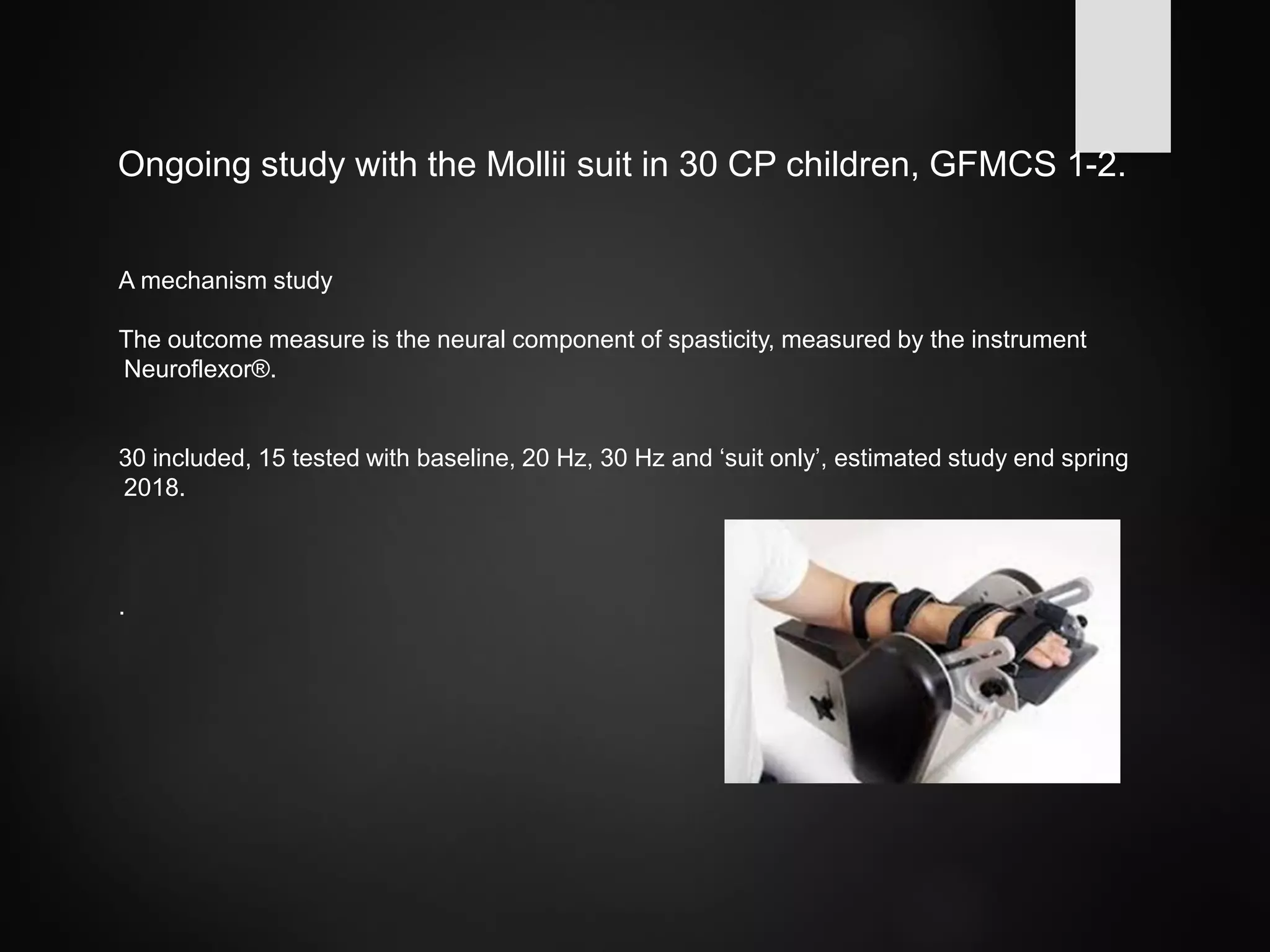
This document discusses spasticity management and Inerventions' product called Mollii, which uses electrical therapy to reduce spasticity and improve motor control for people with conditions like cerebral palsy, stroke, and multiple sclerosis. Mollii is a medical device suit with electrodes that can be individually programmed. Clinical studies in Sweden and Denmark show that Mollii reduces spasticity and facilitates voluntary movement, helping users to live more freely. Inerventions' vision is for Mollii to become a natural first choice for non-invasive spasticity management.