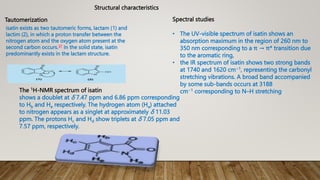
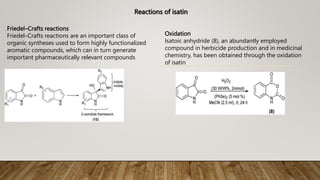
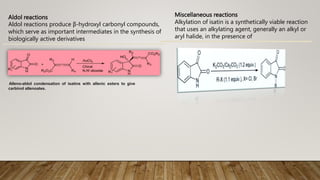
The essay discusses isatin and its derivatives, highlighting their synthesis, reactivity, and biological evaluations. Various synthesis methods such as Sandmeyer, Martinet, Stolle, and Gassman are detailed, along with the biological activities of different isatin derivatives, notably their anti-cancer properties. Additionally, industrial applications of isatin, including its use as a corrosion inhibitor and in dye production, are also addressed, emphasizing its significance in synthetic and medicinal chemistry.