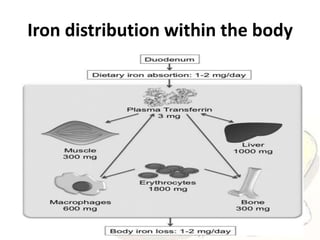
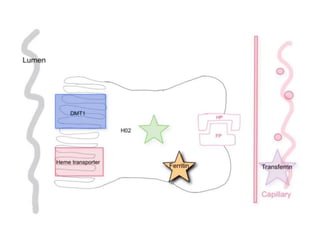
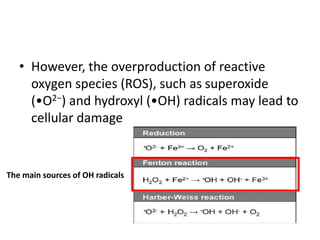
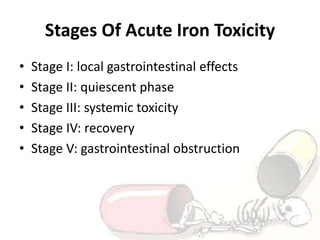
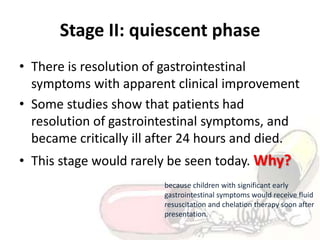
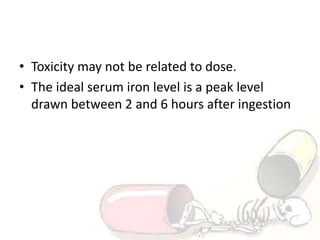
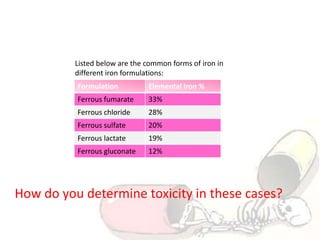
The document discusses iron toxicity, including its chemical properties, epidemiology, sources, absorption in the body, toxicity, pathophysiology of iron poisoning, diagnosis, management, and a case study of iron toxicity in a child. It provides details on the stages of acute iron toxicity, signs and symptoms, laboratory tests, criteria for chelation therapy, and treatment approaches.