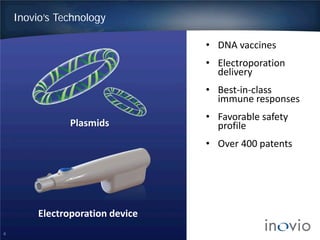
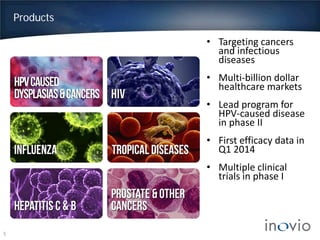
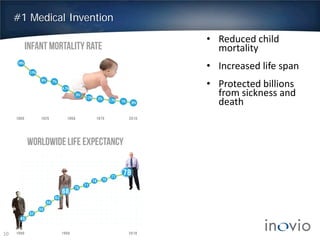
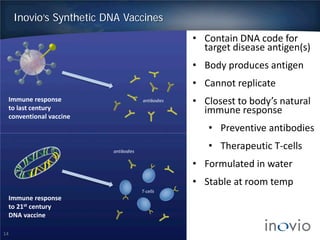
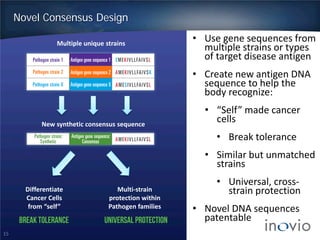
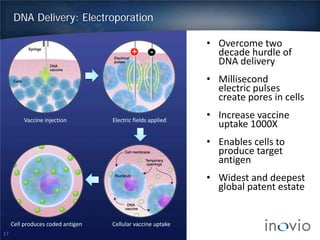
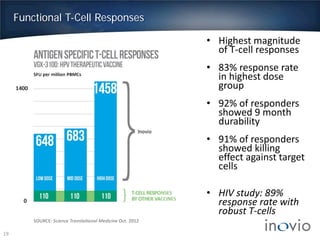
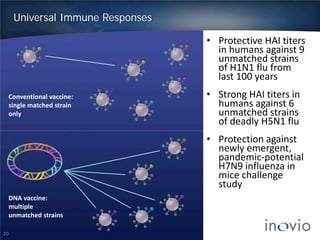
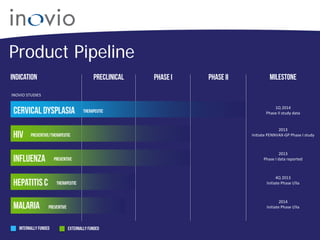
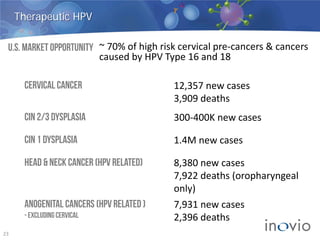
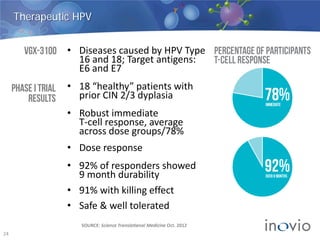
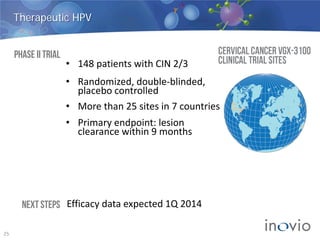
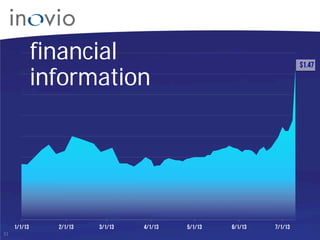
This document summarizes Inovio Pharmaceuticals' DNA vaccine technology and development programs. It discusses Inovio's synthetic DNA vaccines that stimulate both antibody and T-cell immune responses, their electroporation delivery system, and their ability to generate protective responses against multiple unmatched strains of pathogens. The lead program is a therapeutic HPV vaccine in Phase II clinical trials, with efficacy data expected in Q1 2014. Inovio has a pipeline of DNA vaccine programs for cancers and infectious diseases, and pursues partnerships, grants, and clinical trial sponsorships to fund development.