This document summarizes information presented by Dr. J. Joseph Kim, President and CEO of Inovio Pharmaceuticals, Inc. It discusses Inovio's DNA immunotherapy technology, clinical programs, partnerships, leadership, and upcoming value drivers. Key points include:
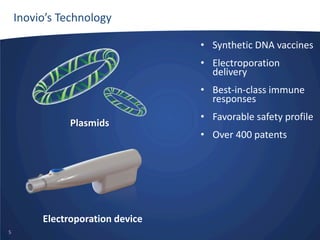
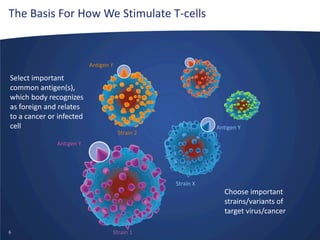
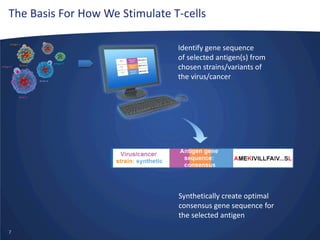
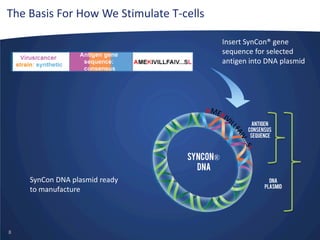
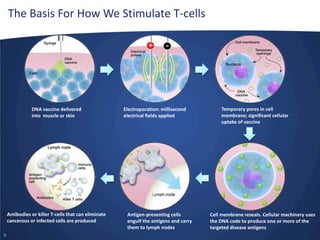
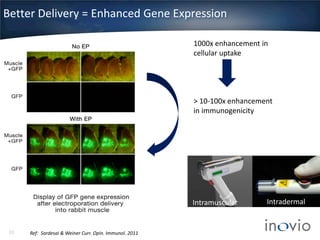
- Inovio is developing DNA-based immunotherapies delivered using electroporation for cancers and infectious diseases.
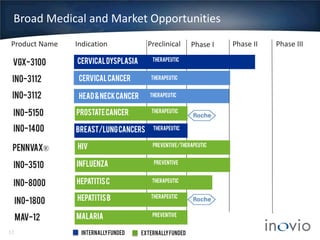
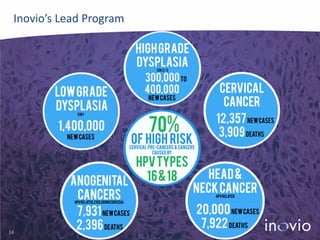
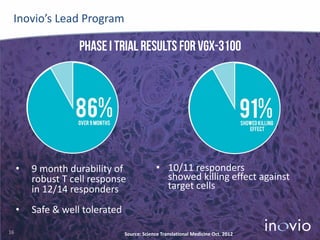
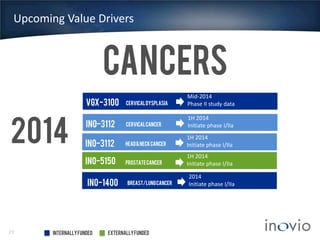
- Their lead program, VGX-3100 for HPV-related cancers, has shown strong T-cell responses and is in Phase II trials with efficacy data expected in mid-2014.
- Inovio has partnerships with Roche for prostate cancer and hepatitis B therapies and external funding to support several clinical programs.