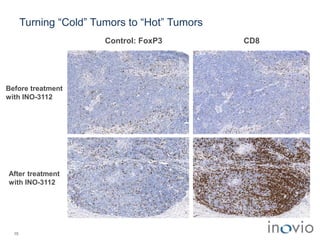
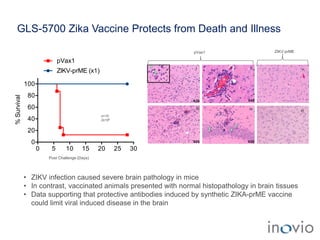
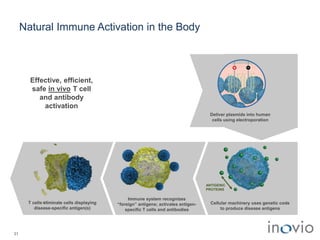
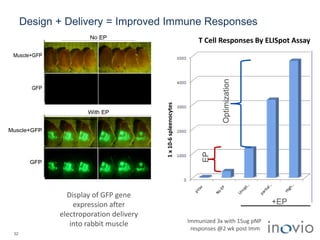
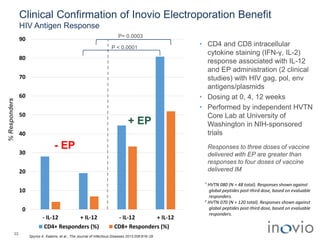
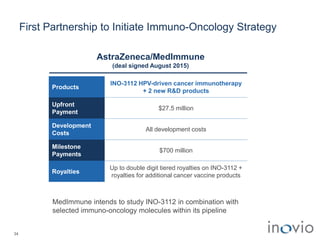
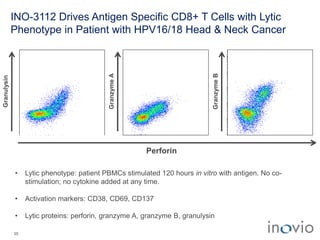
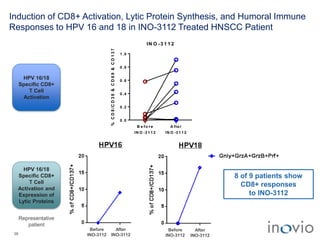
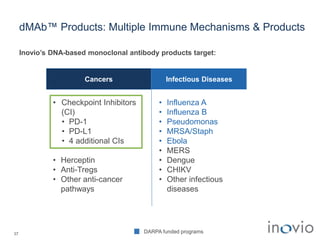
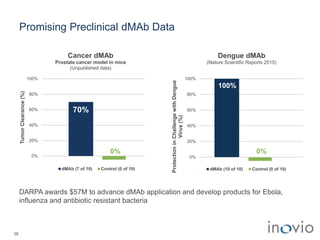
The document summarizes Inovio Pharmaceuticals' immunotherapy platform and development pipeline. Key points include:
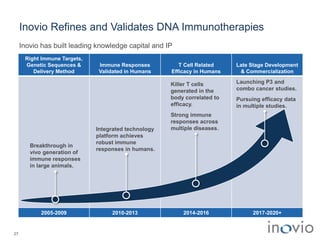
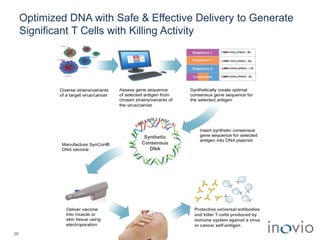
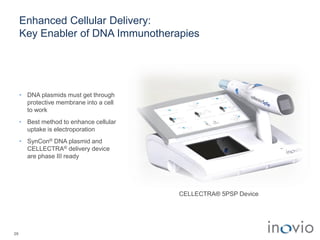
1) Inovio is developing DNA-based immunotherapies and vaccines to treat cancers and infectious diseases using its SynCon platform and CELLECTRA delivery device.
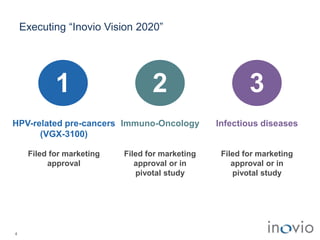
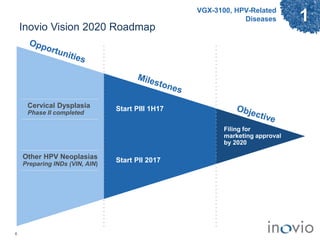
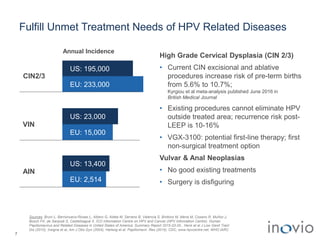
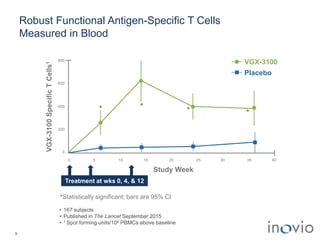
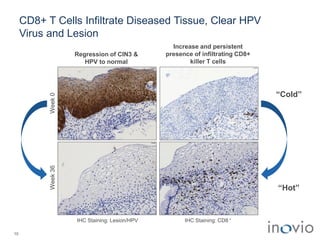
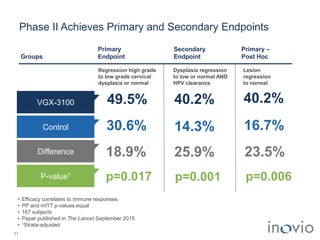
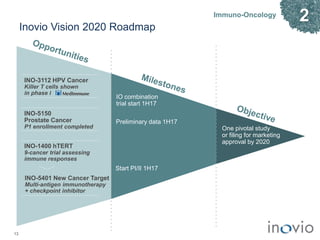
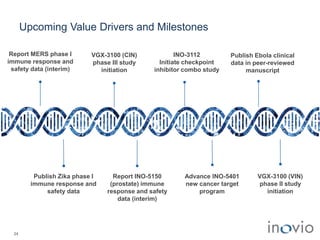
2) Its lead product, VGX-3100 for HPV-related diseases, achieved efficacy in a phase II trial and plans to start a phase III trial in 2017.
3) Inovio is also developing immunotherapies for immuno-oncology including combination trials with checkpoint inhibitors starting in 2017.
4) It has infectious disease vaccines in development for Ebola, MERS, Zika