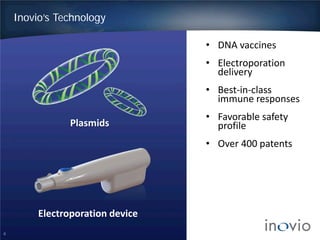
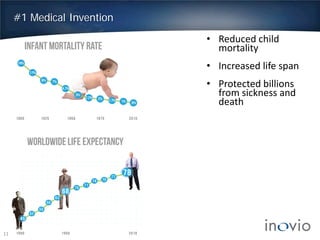
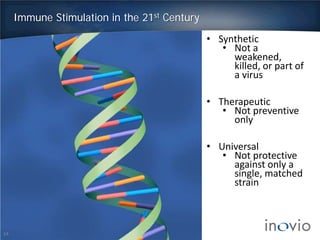
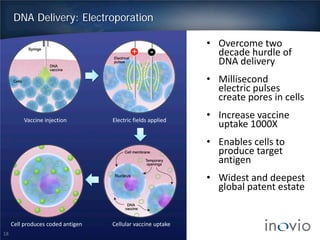
This document summarizes the work of Dr. J. Joseph Kim and Inovio Pharmaceuticals to revolutionize vaccines. It discusses Inovio's DNA vaccine technology which uses plasmids and electroporation to efficiently stimulate the immune system. Key points include:
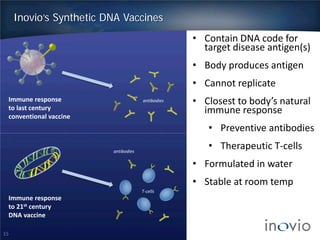
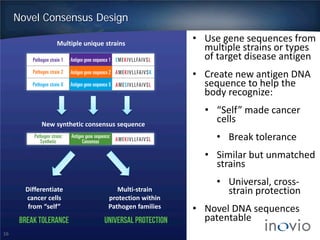
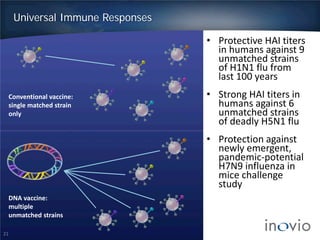
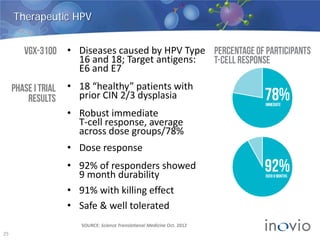
- DNA vaccines can provide universal and therapeutic immune responses against multiple strains of diseases like HPV, cancer, HIV, influenza.
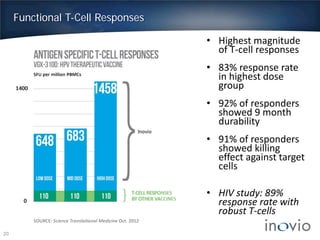
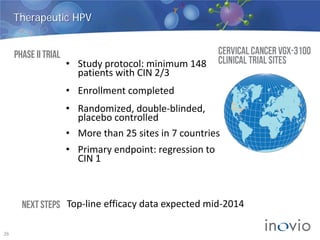
- Clinical trials show DNA vaccines generate best-in-class T-cell and antibody responses.
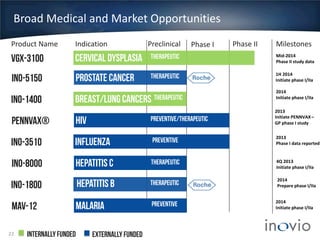
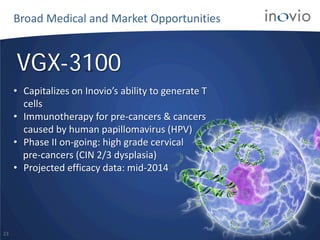
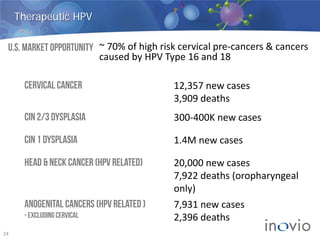
- The company is developing vaccines for cancers, HPV, HIV, influenza, hepatitis through partnerships and clinical trials.
- A partnership with Roche will develop prostate cancer and hepatitis B vaccines.