Embed presentation
Downloaded 19 times

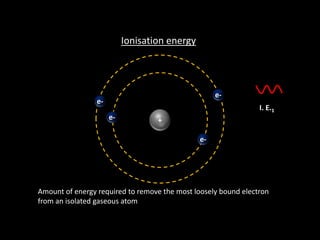
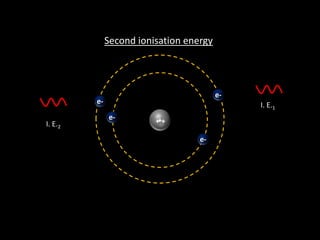
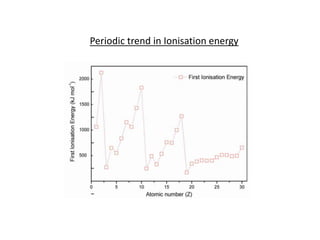


Ionisation energy is defined as the energy required to remove the most loosely bound electron from an isolated gaseous atom. Key factors influencing ionisation energy include atomic size, the charge on the atom, and the effectiveness of inner electron shells in screening nuclear charge. The periodic trend shows that ionisation energy decreases down a group and increases across a period.





