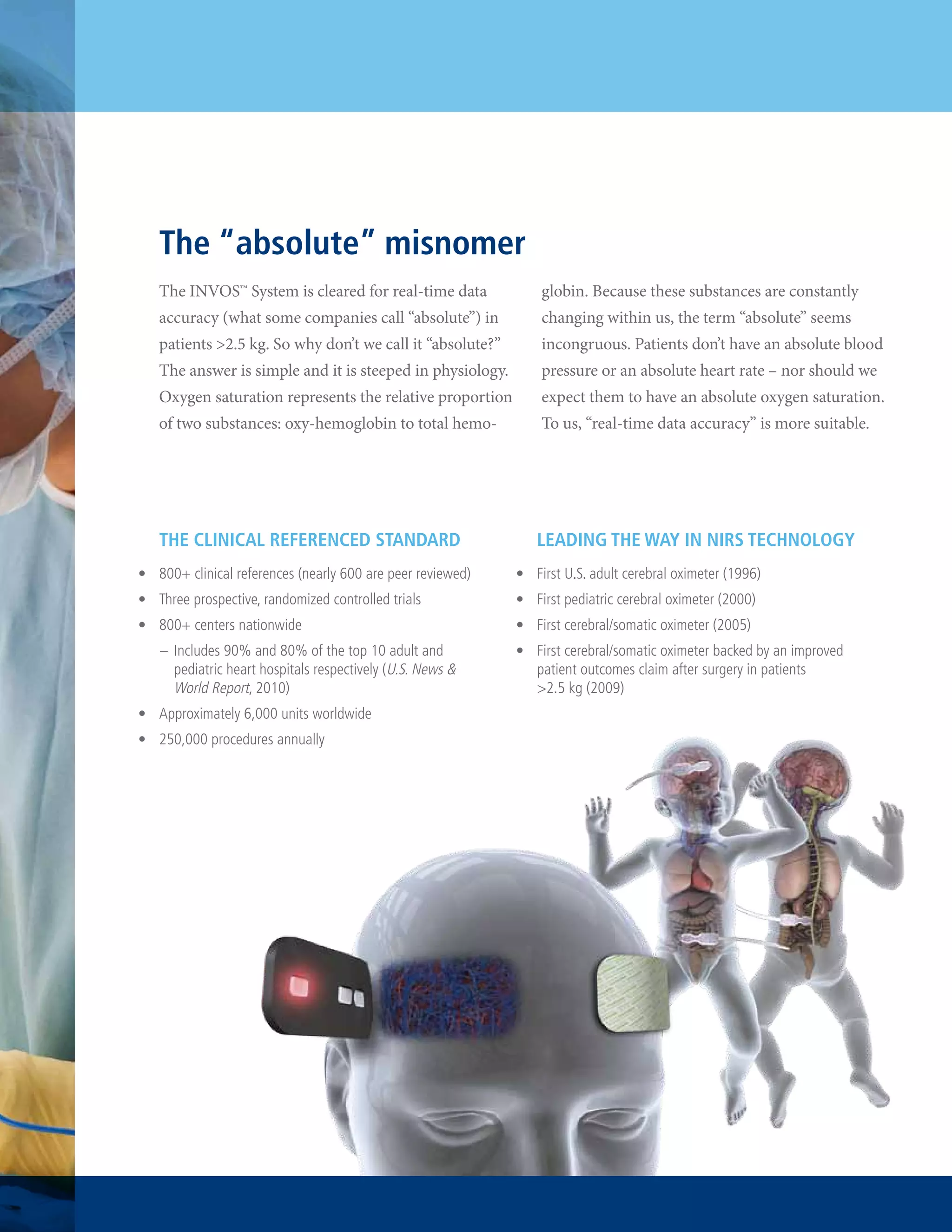
The document summarizes the advantages of the INVOSTM System, a sixth generation cerebral/somatic oximeter for monitoring patient oxygen levels. It highlights that the INVOSTM System is the only oximeter backed by evidence of improved patient outcomes, with over 800 clinical references including 600 peer-reviewed studies. It also is the only system that enables simultaneous four-channel brain and body monitoring and has a proven 14-year history of accurate and expanded clinical applications.