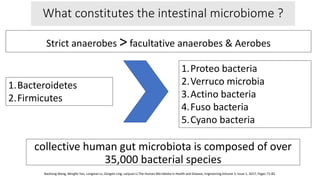
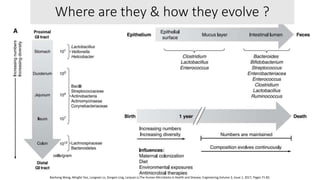
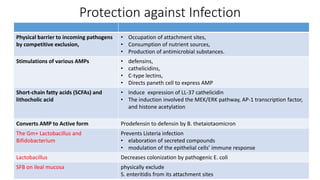
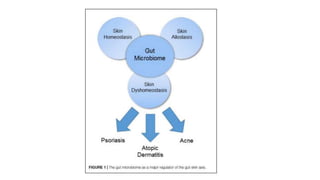
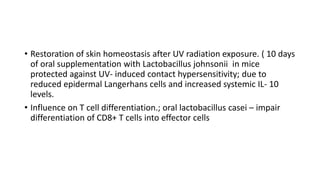
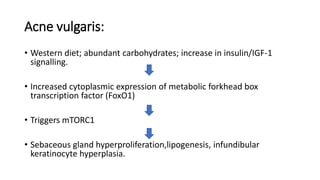
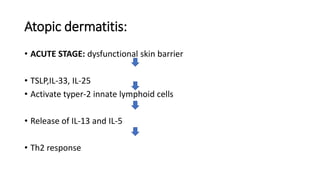
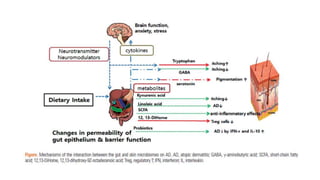
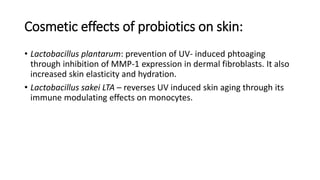
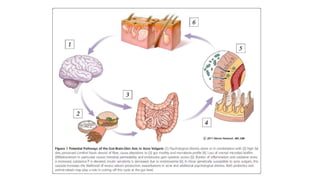
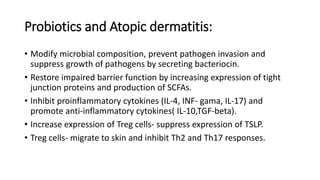
The document discusses the relationship between the intestinal microbiome and various skin disorders, emphasizing the bidirectional communication between gut and skin health. It highlights the roles of specific gut microbes in modulating immune responses and their impact on conditions like acne, atopic dermatitis, psoriasis, and rosacea. Additionally, it explores the potential benefits of probiotics and prebiotics in restoring skin homeostasis and treating skin-related conditions.