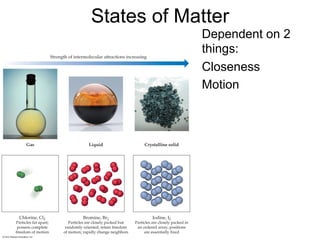
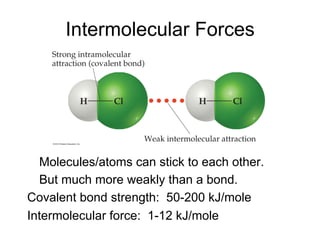
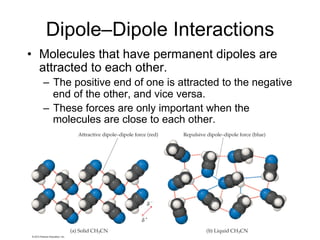
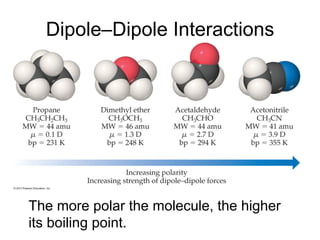
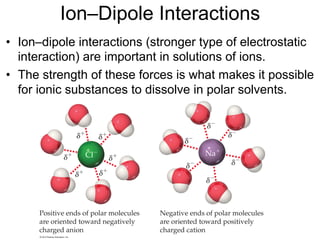
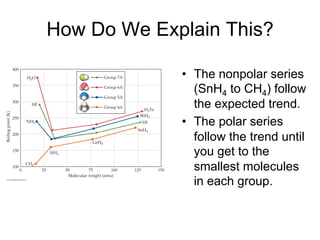
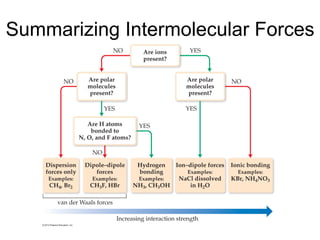
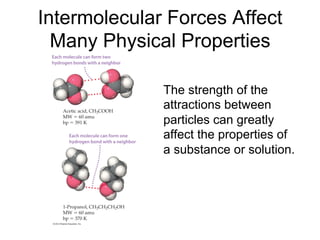
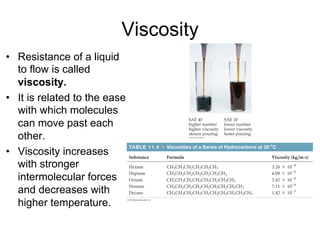
1) Intermolecular forces are weak attractions between molecules that depend on factors like temperature, pressure, and molecular polarity. These forces include dipole-dipole interactions, hydrogen bonding, and London dispersion forces.
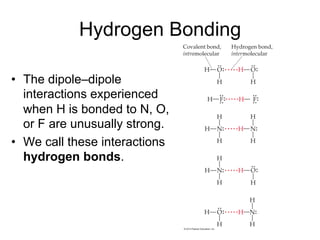
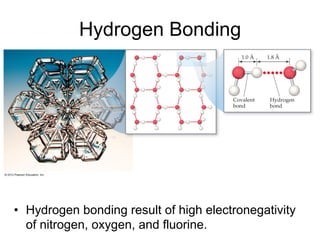
2) Dipole-dipole interactions occur between polar molecules with partial charges, while hydrogen bonding is a stronger form of dipole-dipole interaction between hydrogen and highly electronegative atoms.
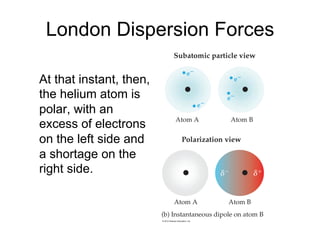
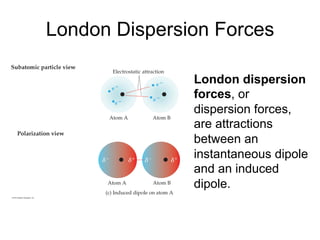
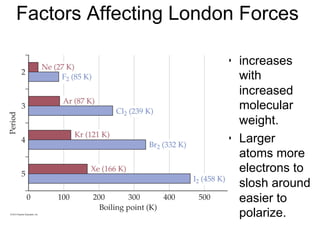
3) London dispersion forces exist in all molecules due to the instantaneous dipoles formed from fluctuations in the electron cloud. They increase with molecular size and polarizability.