The document discusses different types of intermolecular forces:
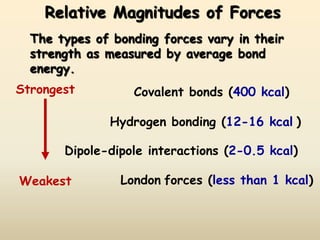
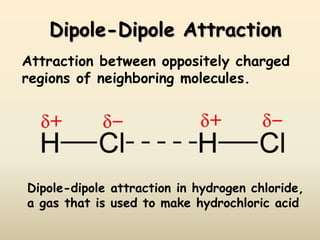
1) Hydrogen bonding, dipole-dipole interactions, and London dispersion forces are the main types of intermolecular forces that determine whether a substance is a solid, liquid or gas.
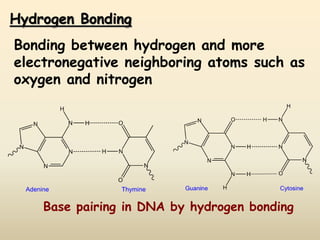
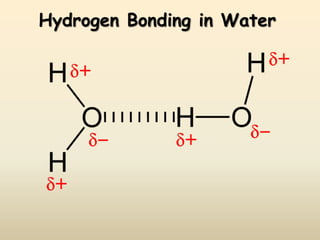
2) Hydrogen bonding is the strongest intermolecular force and occurs between hydrogen and more electronegative atoms like oxygen and nitrogen.
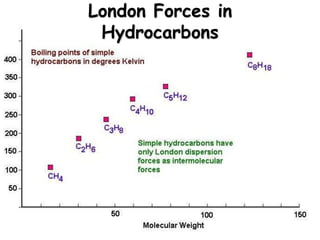
3) London dispersion forces are the weakest intermolecular forces and are the only forces present between nonpolar molecules.