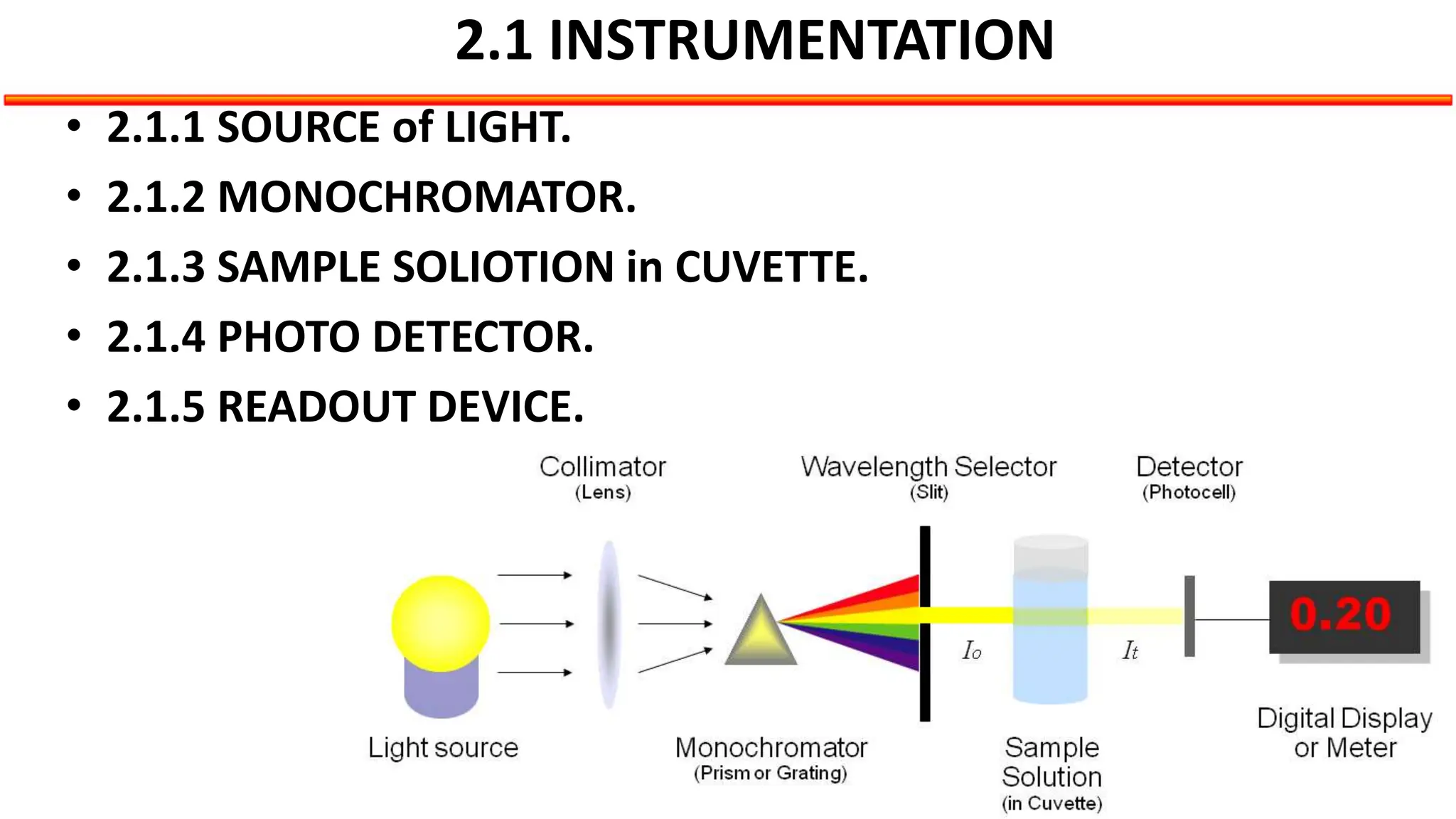
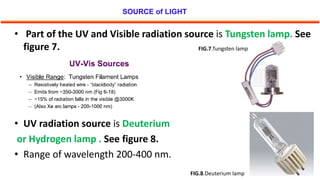
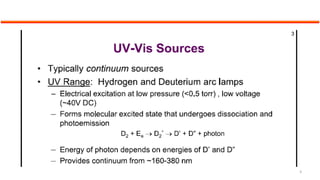
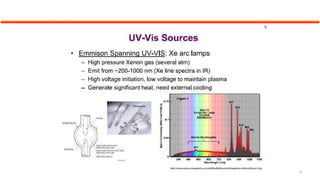
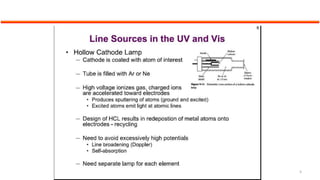
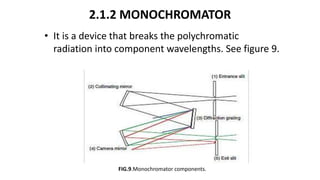
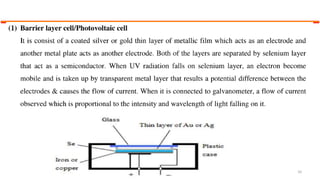
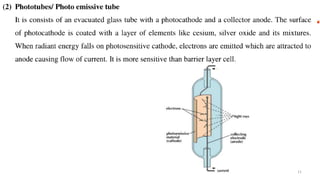
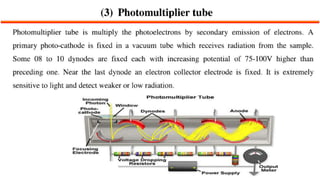
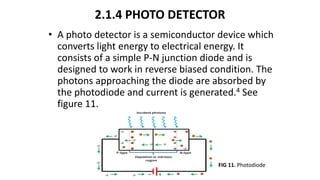
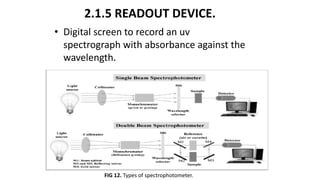
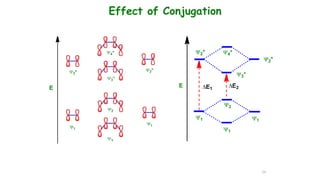
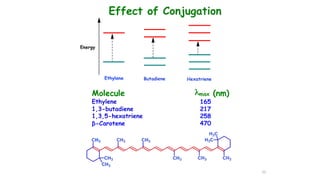
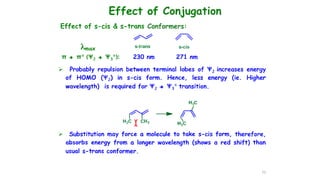
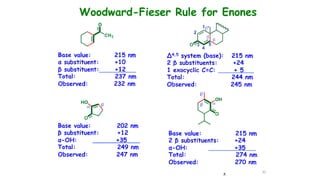
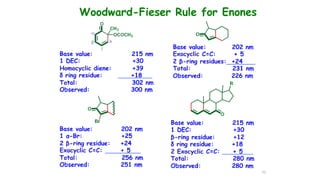
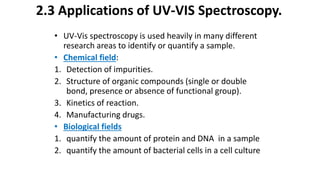
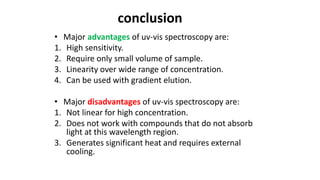
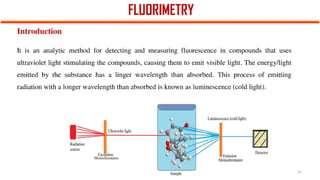
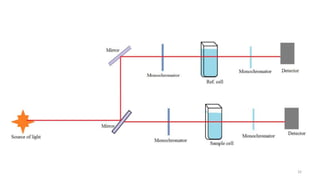
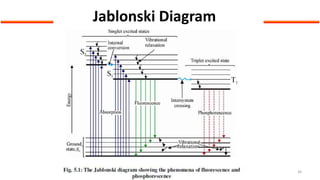
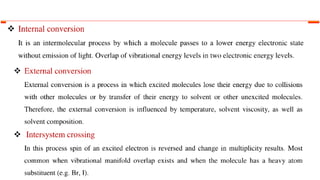
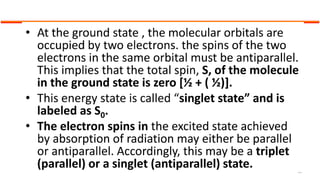
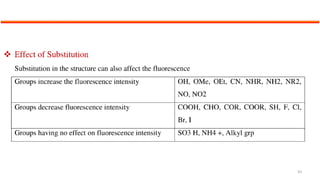
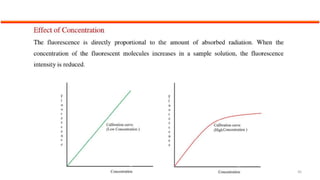
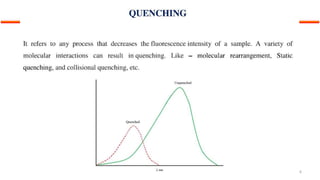
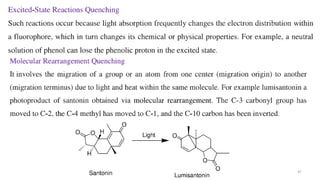
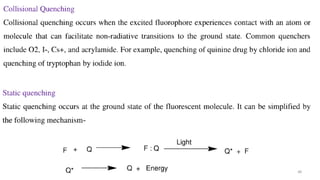
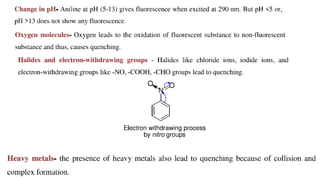
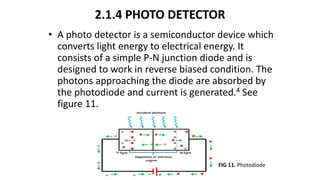
This document describes the components and functioning of UV-Vis spectroscopy instrumentation. It discusses the light source, monochromator, sample cuvette, photodetector, and readout device. The light source is typically a tungsten lamp or deuterium lamp. The monochromator separates polychromatic light into individual wavelengths using an entrance slit, collimating mirror, diffraction grating, focusing mirror, and exit slit. Samples are contained in quartz or glass cuvettes. The photodetector converts light energy to electrical signals. Absorbance data is displayed on a digital readout screen.