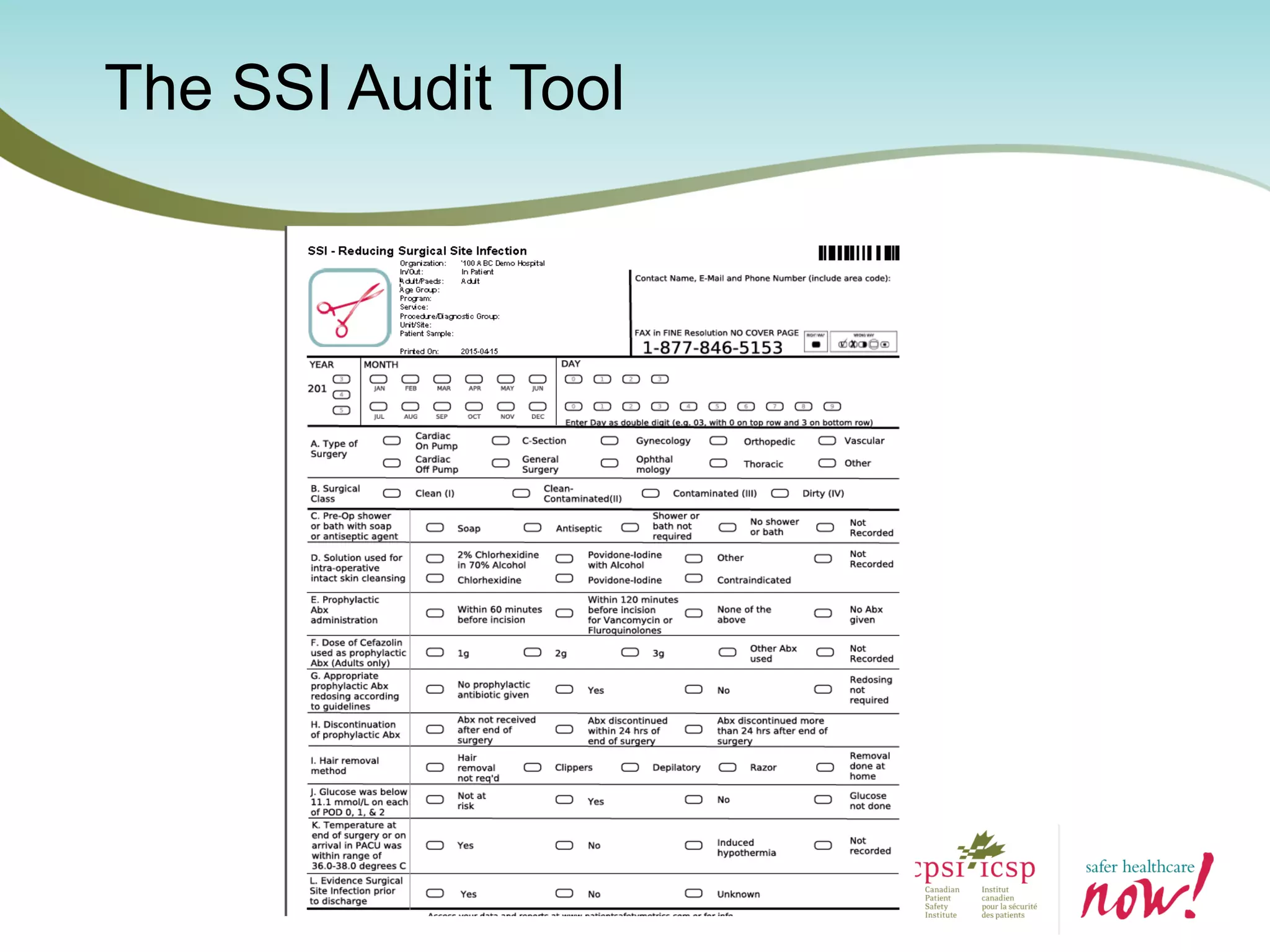
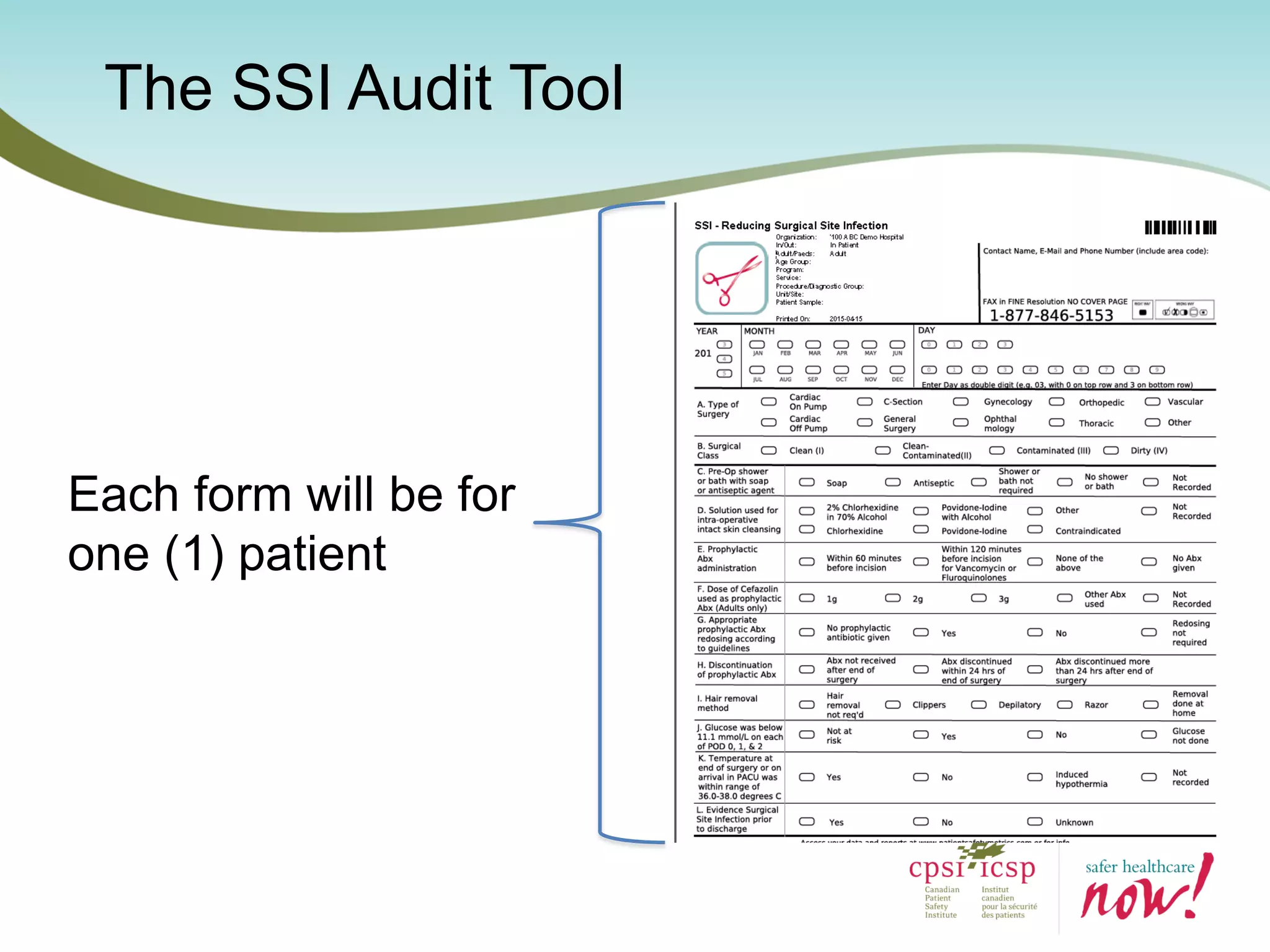
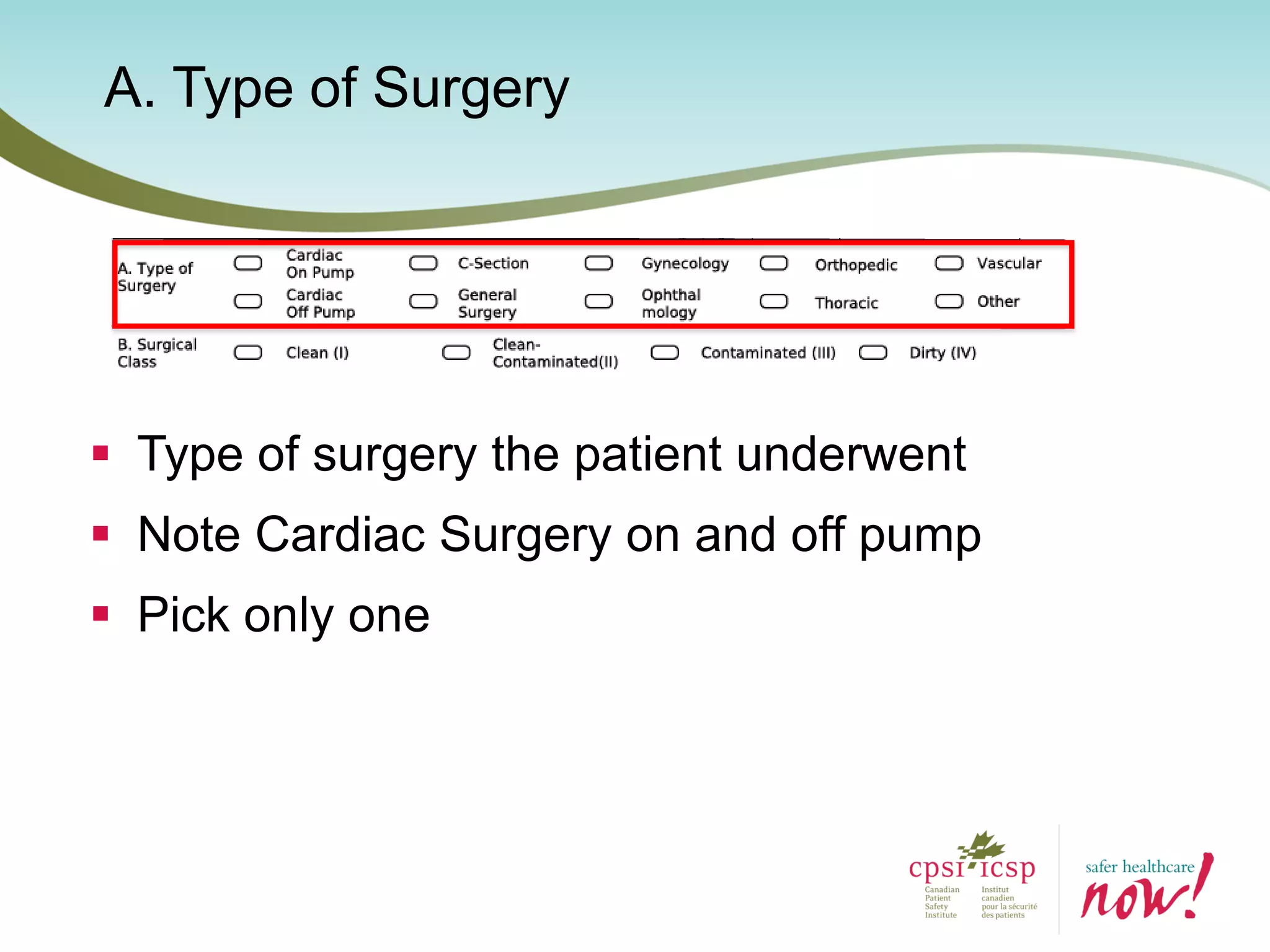
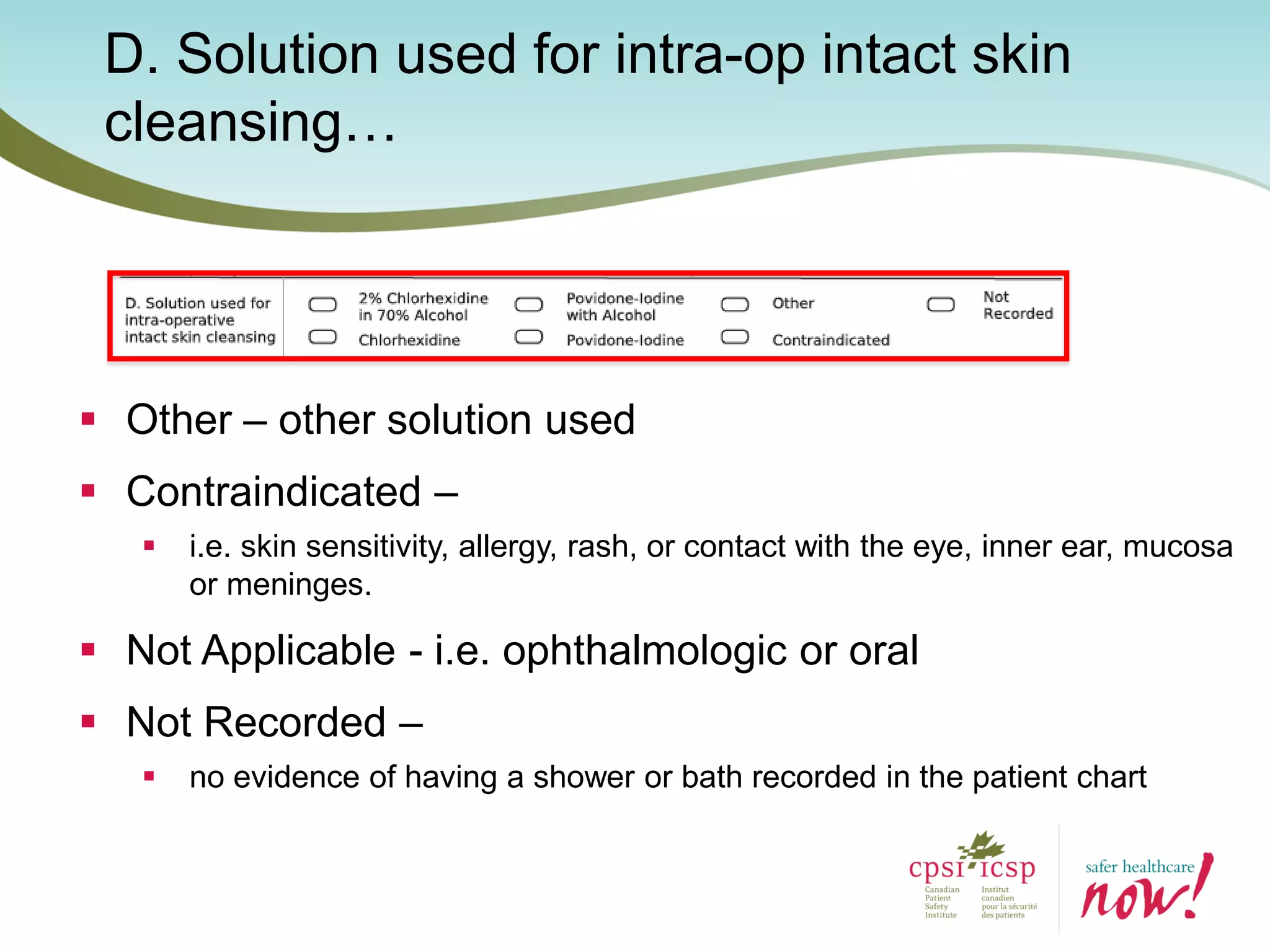
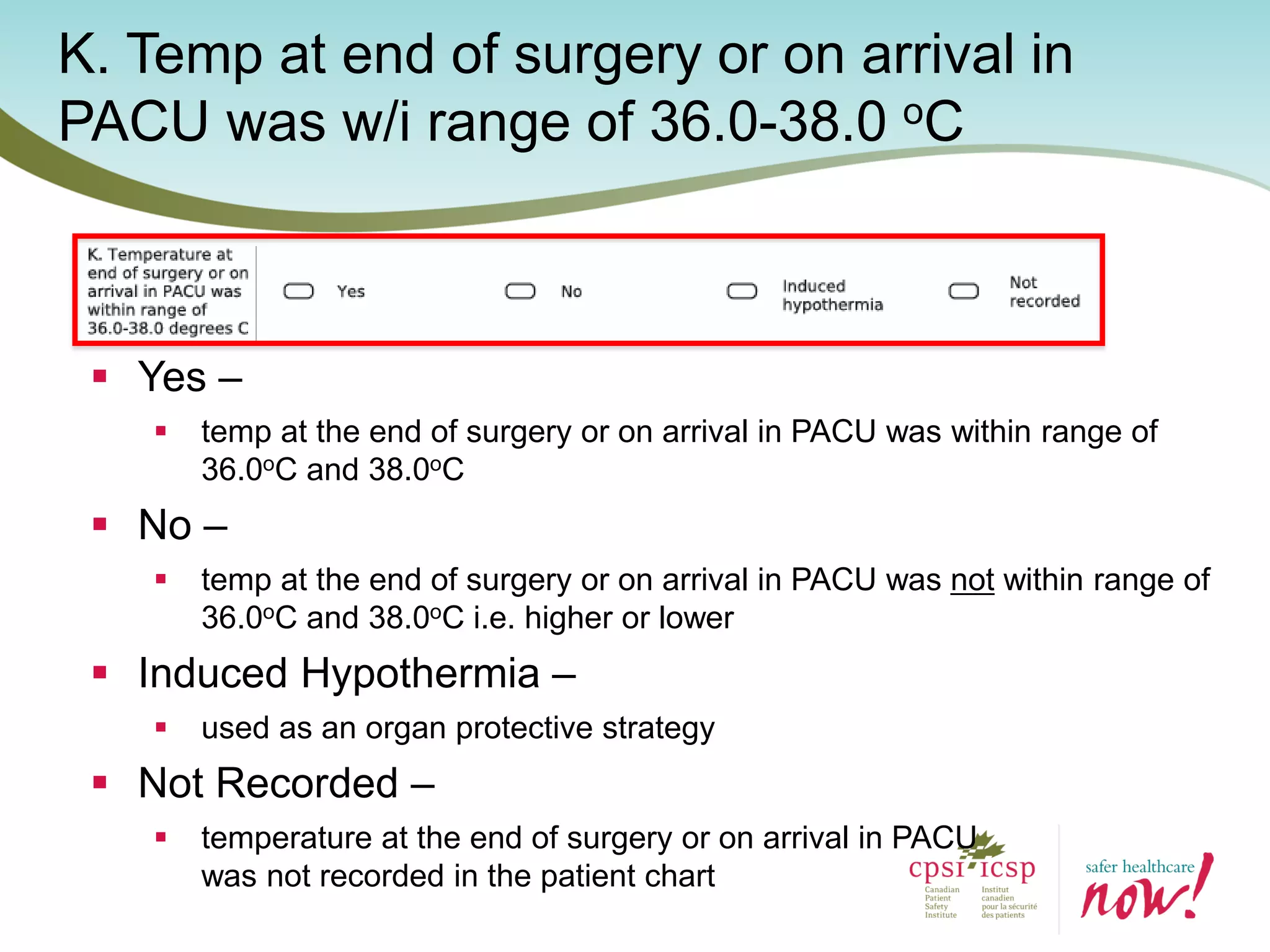
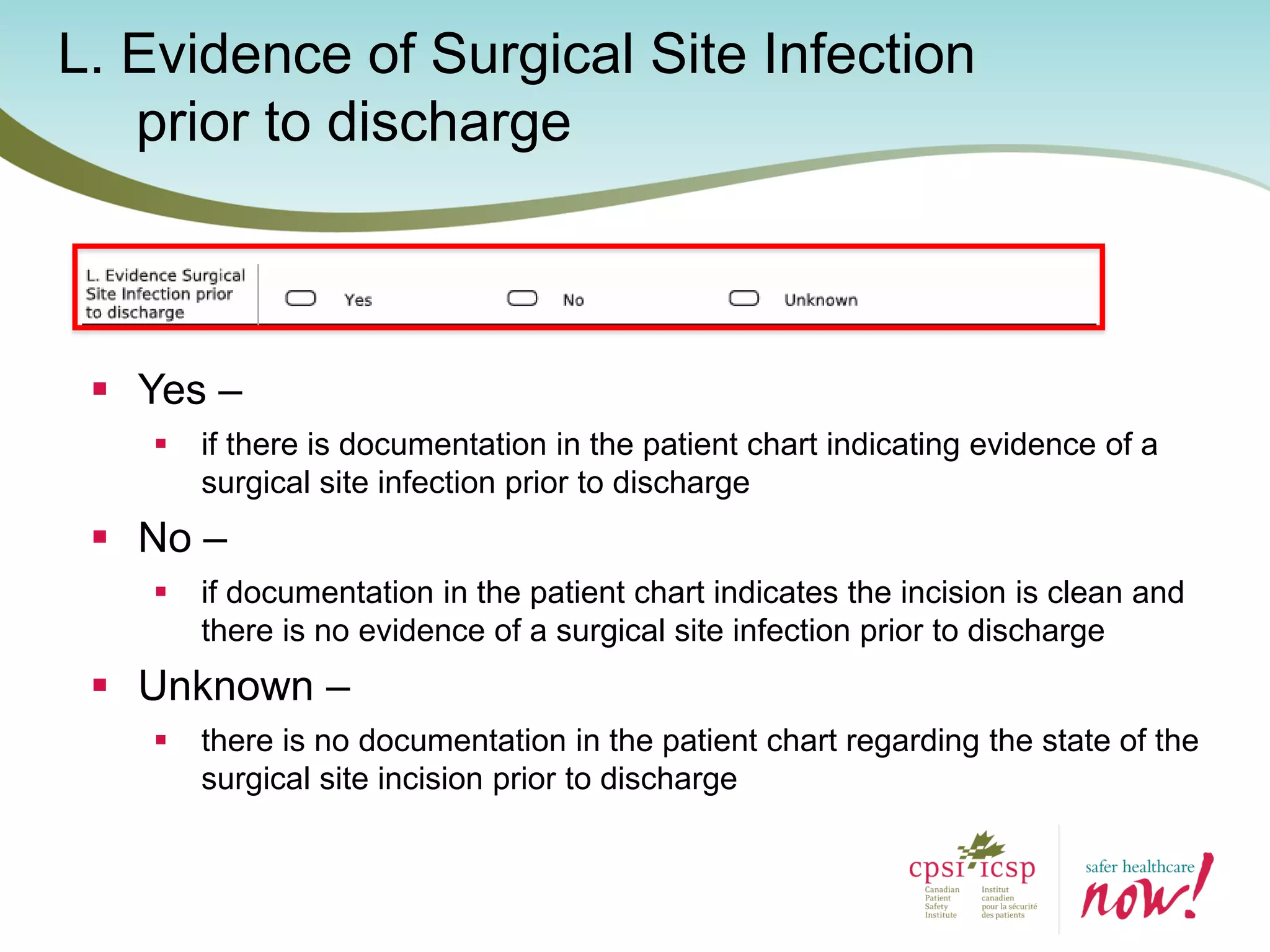
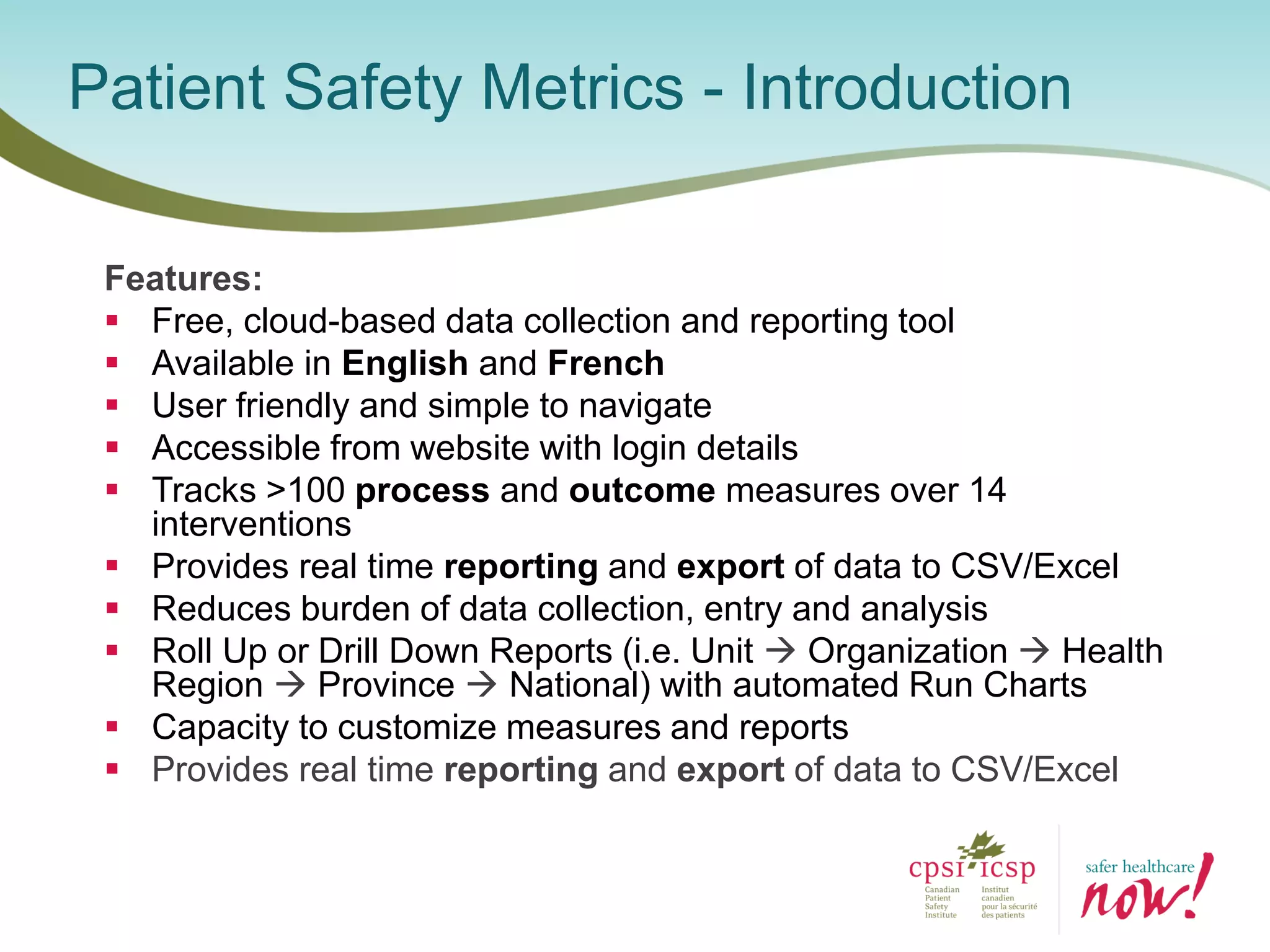
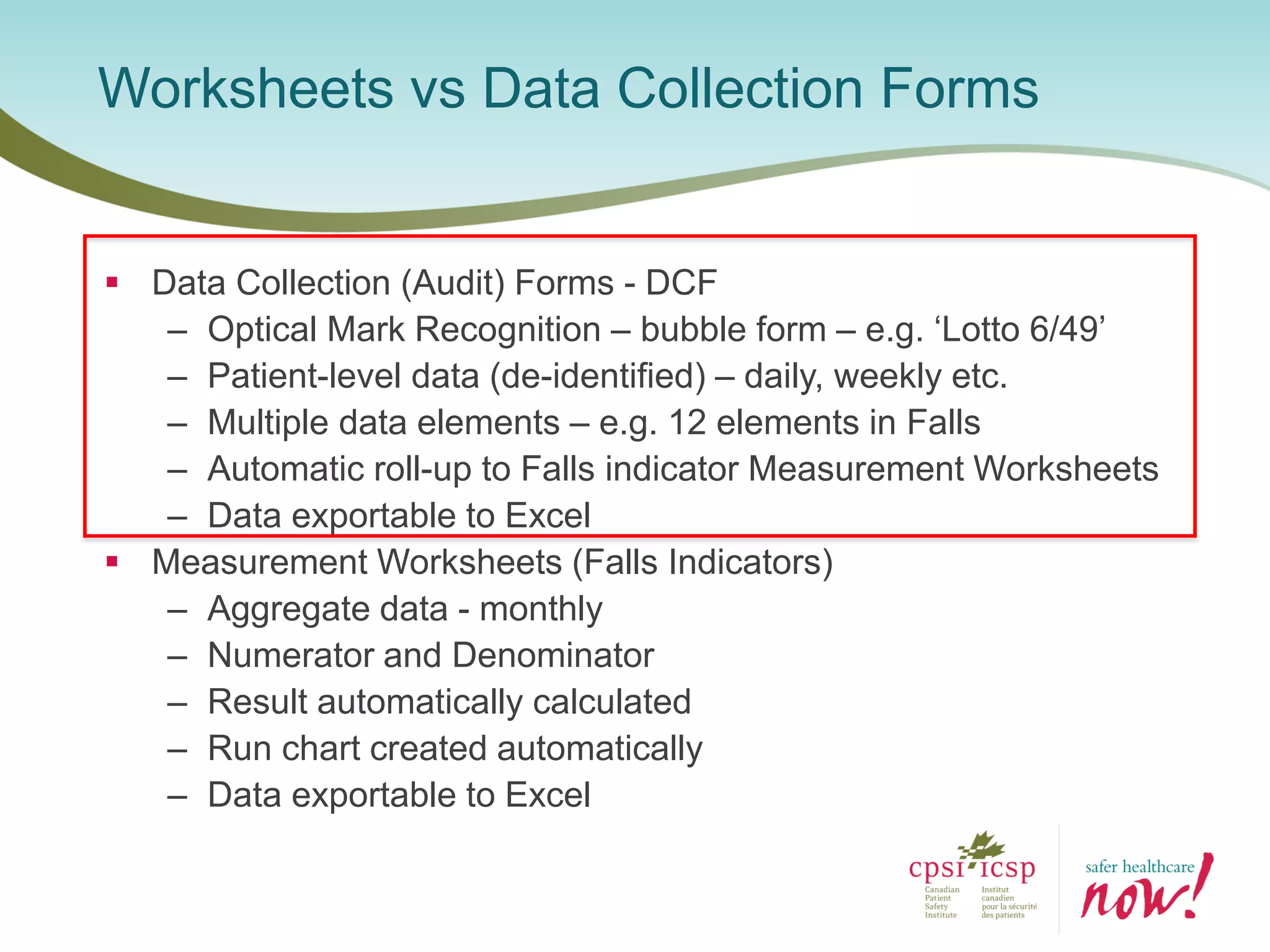
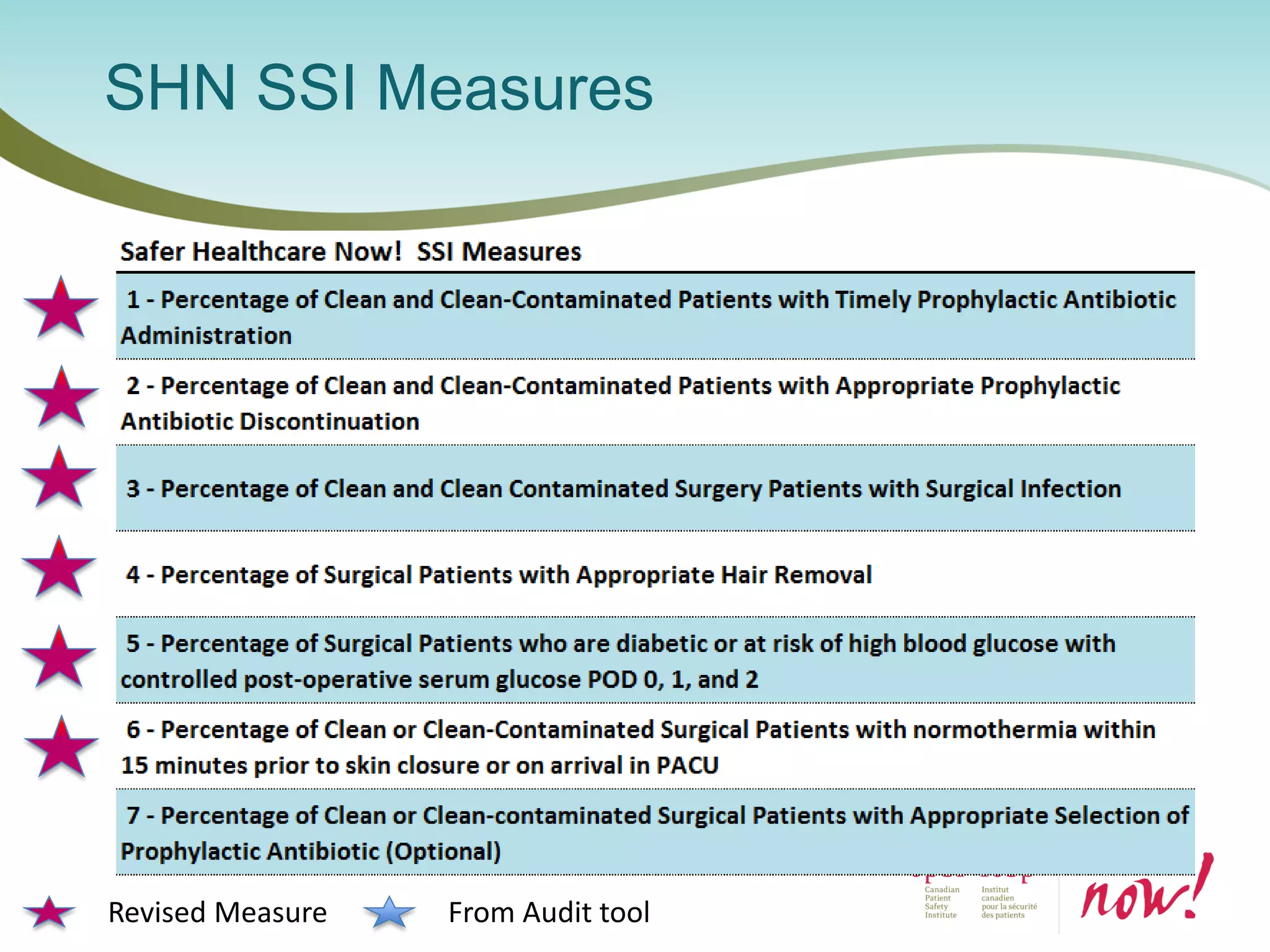
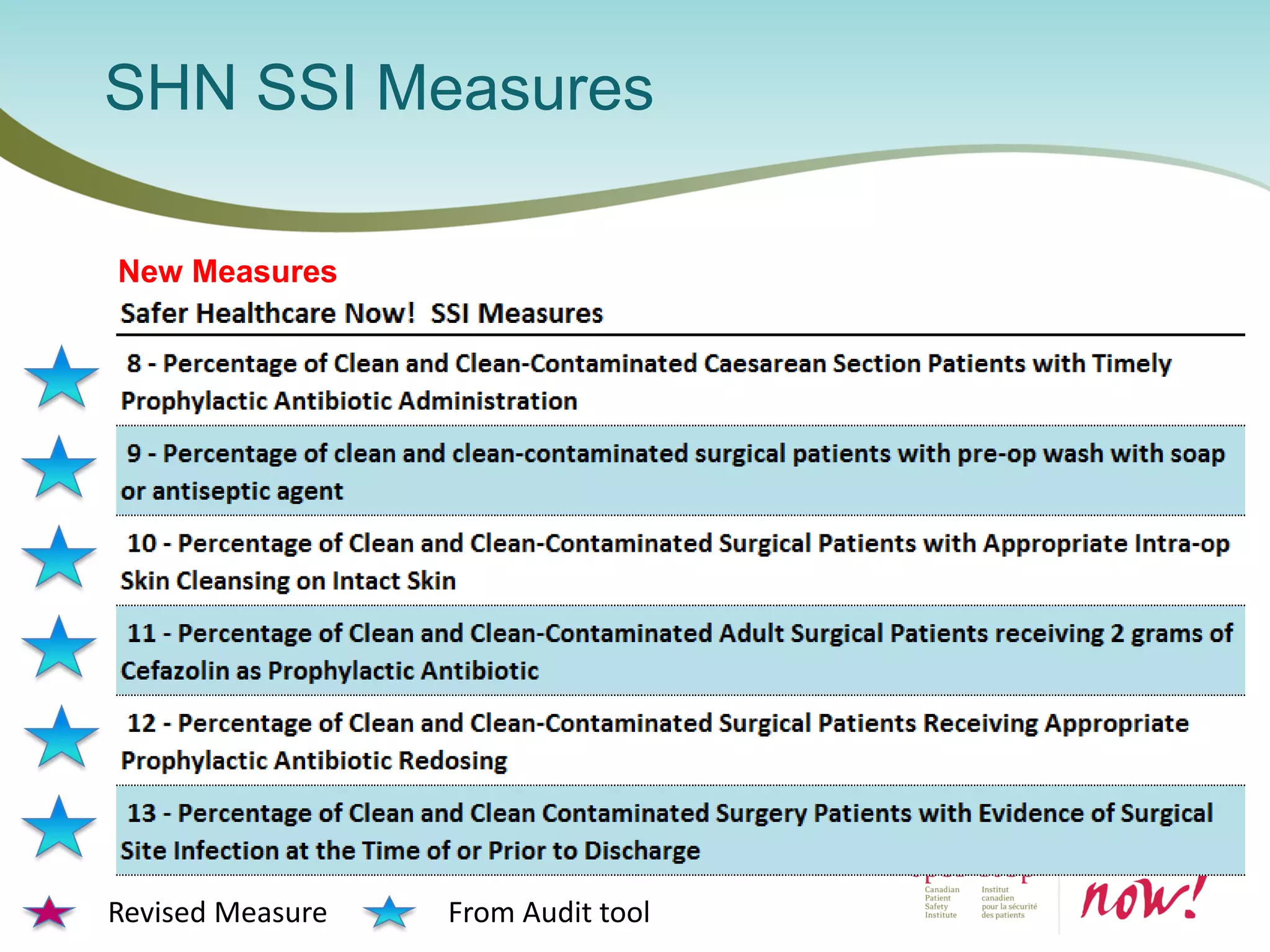
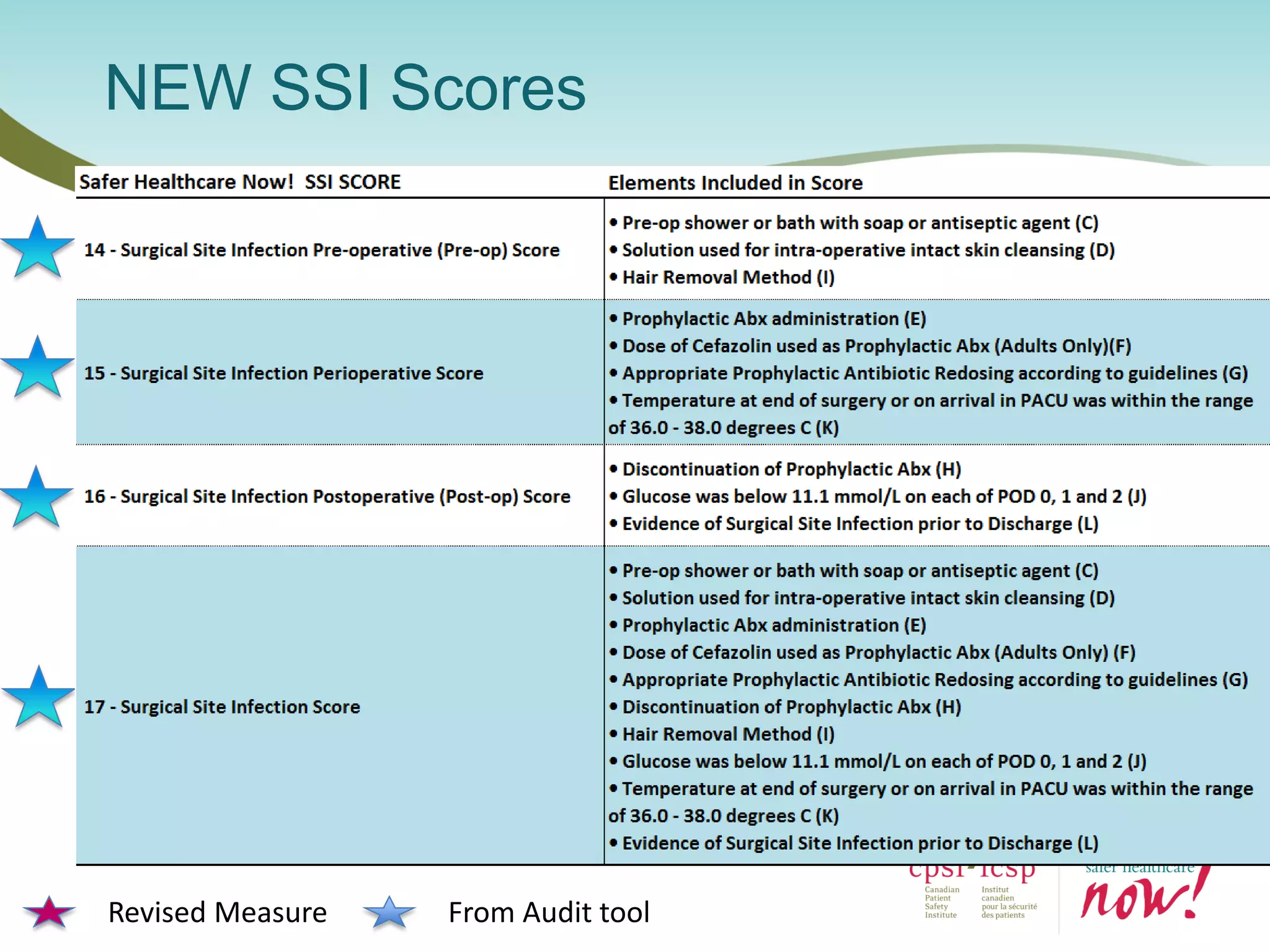
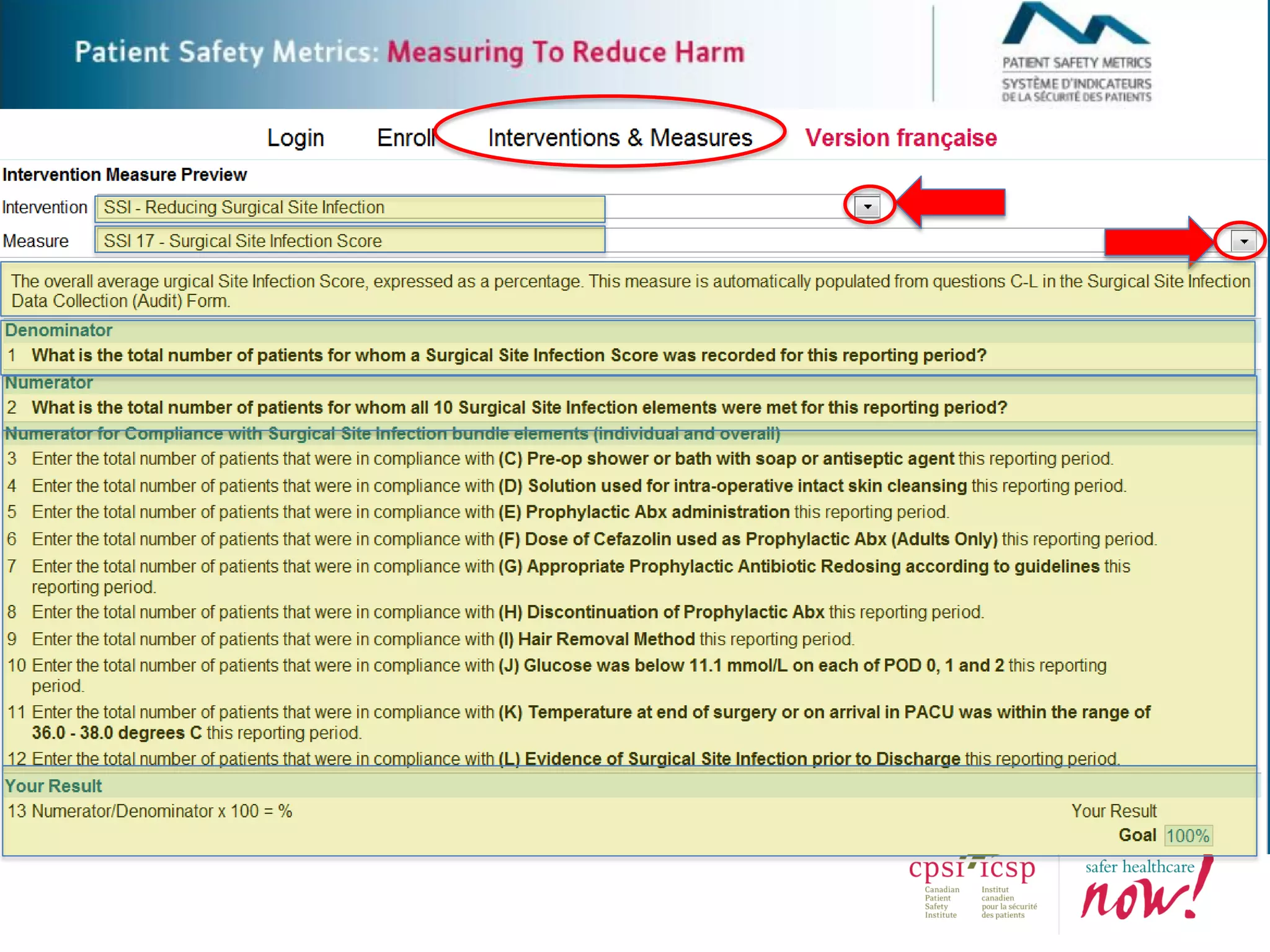
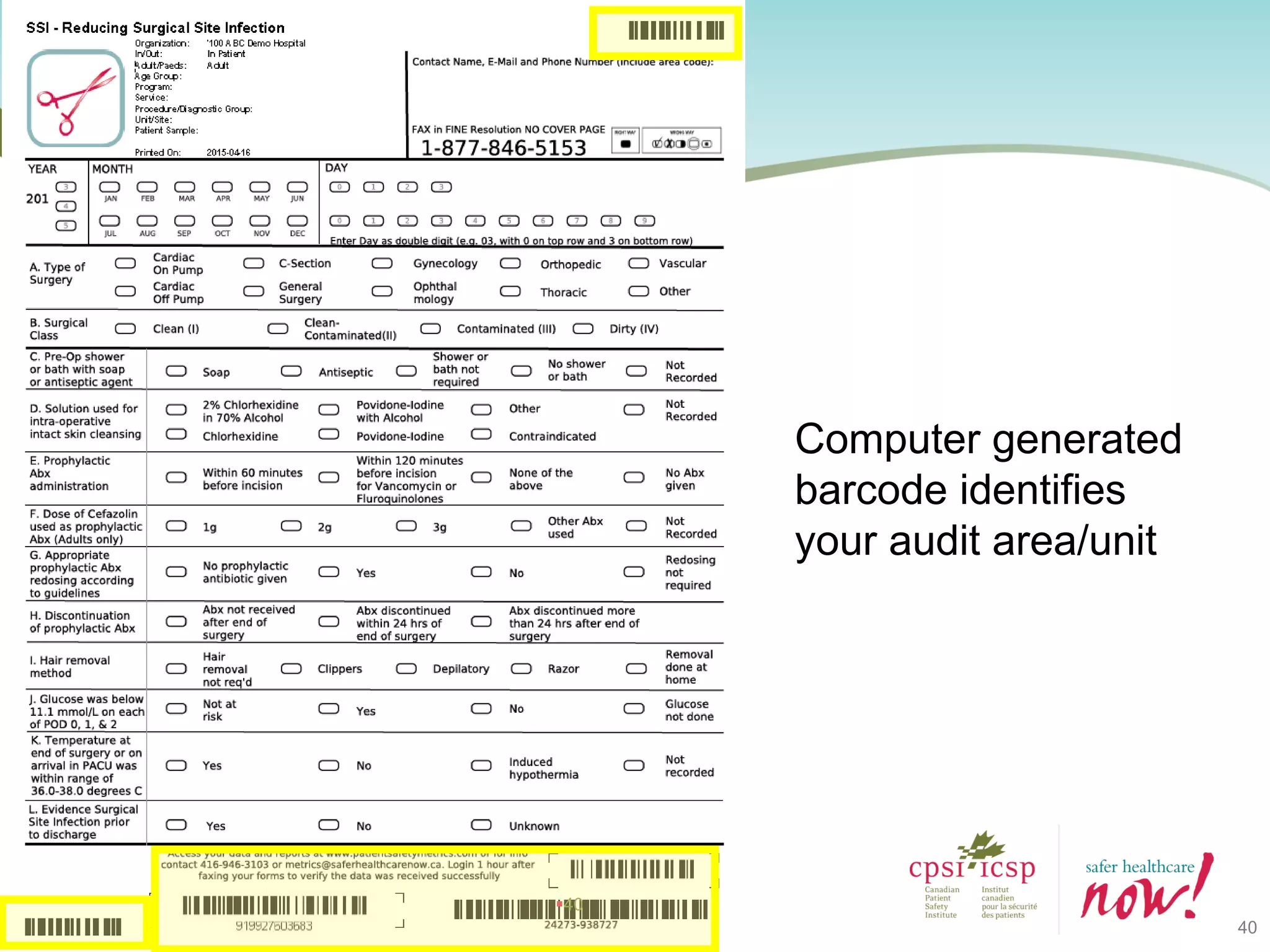
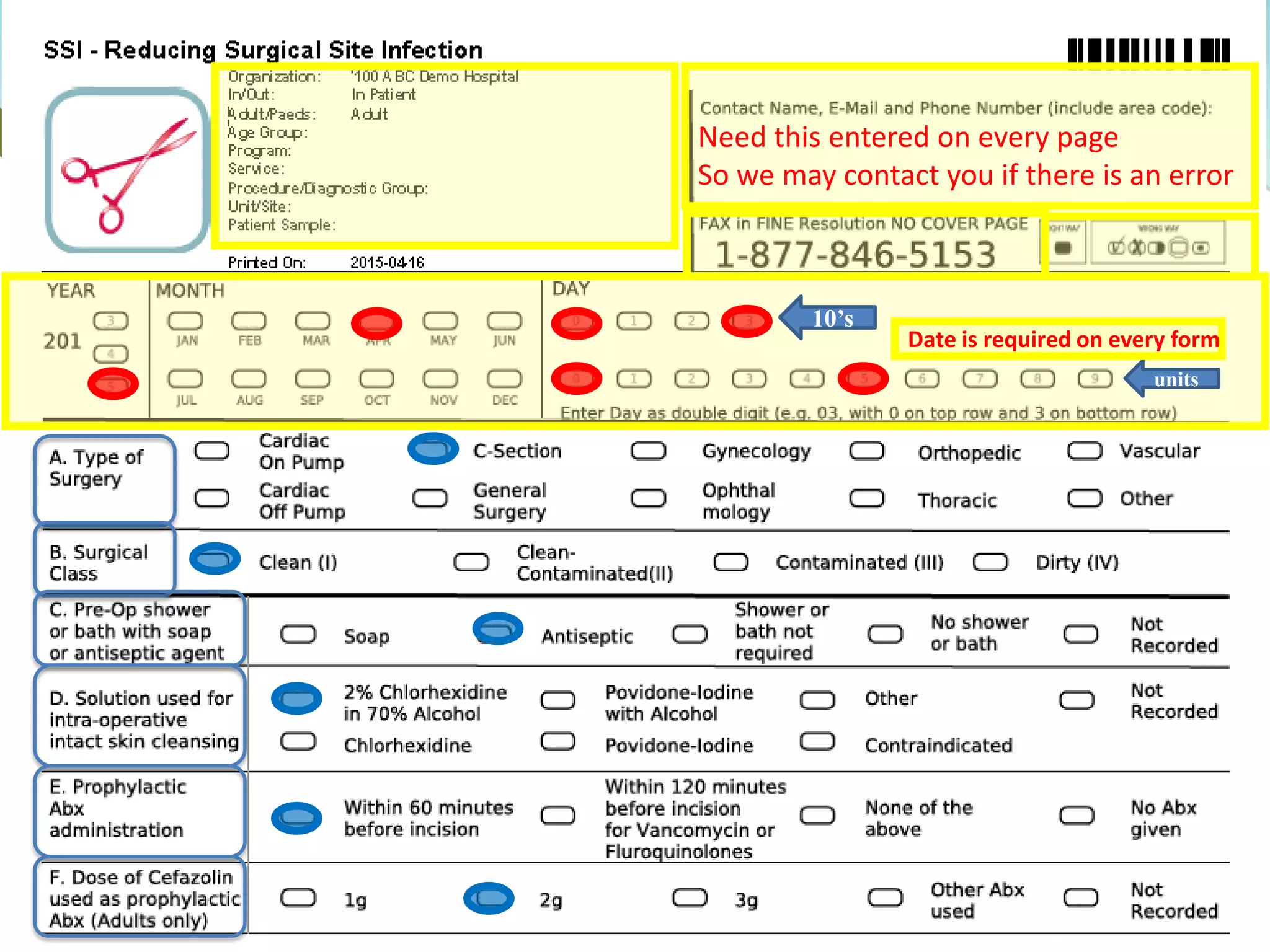
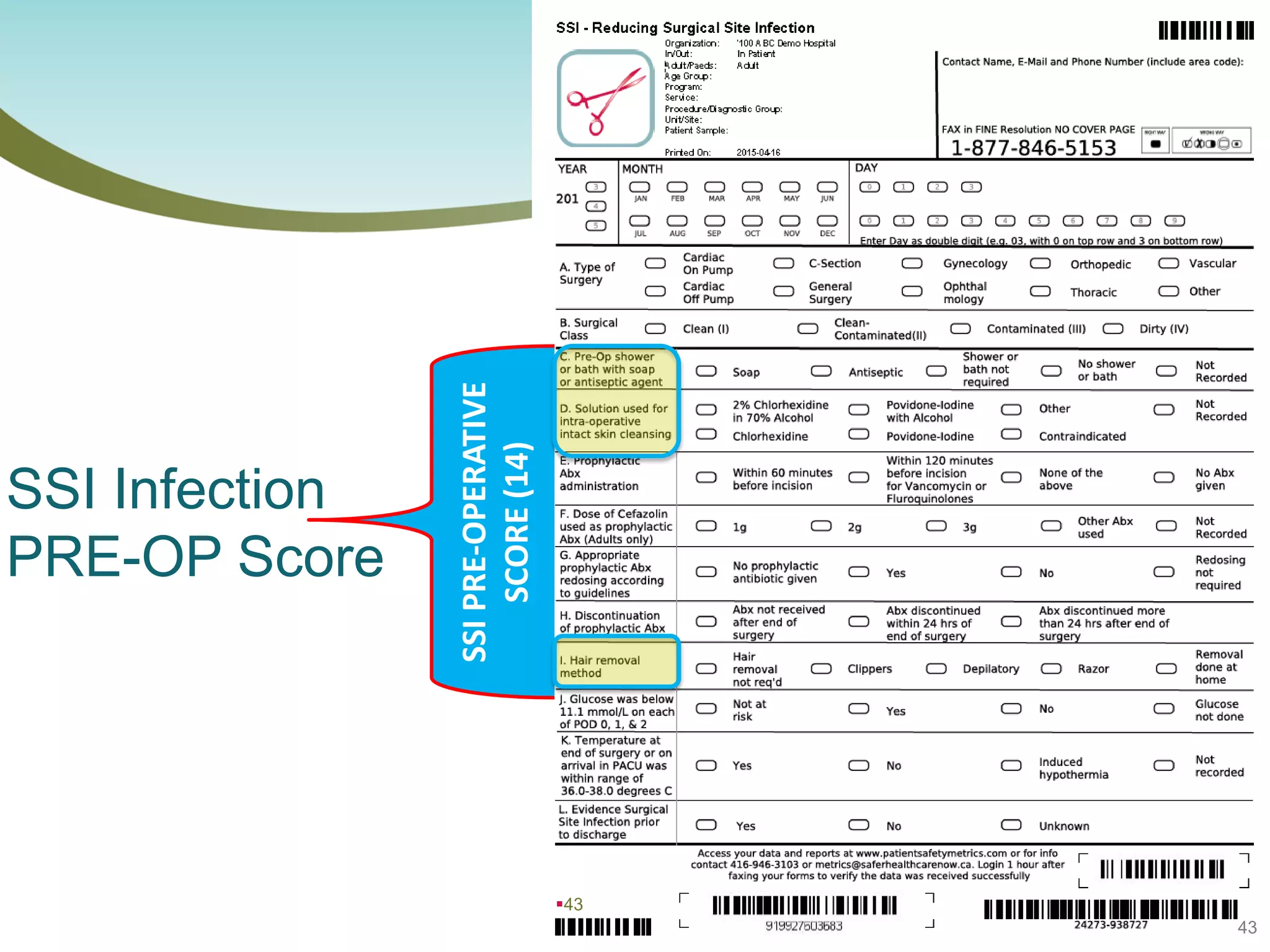
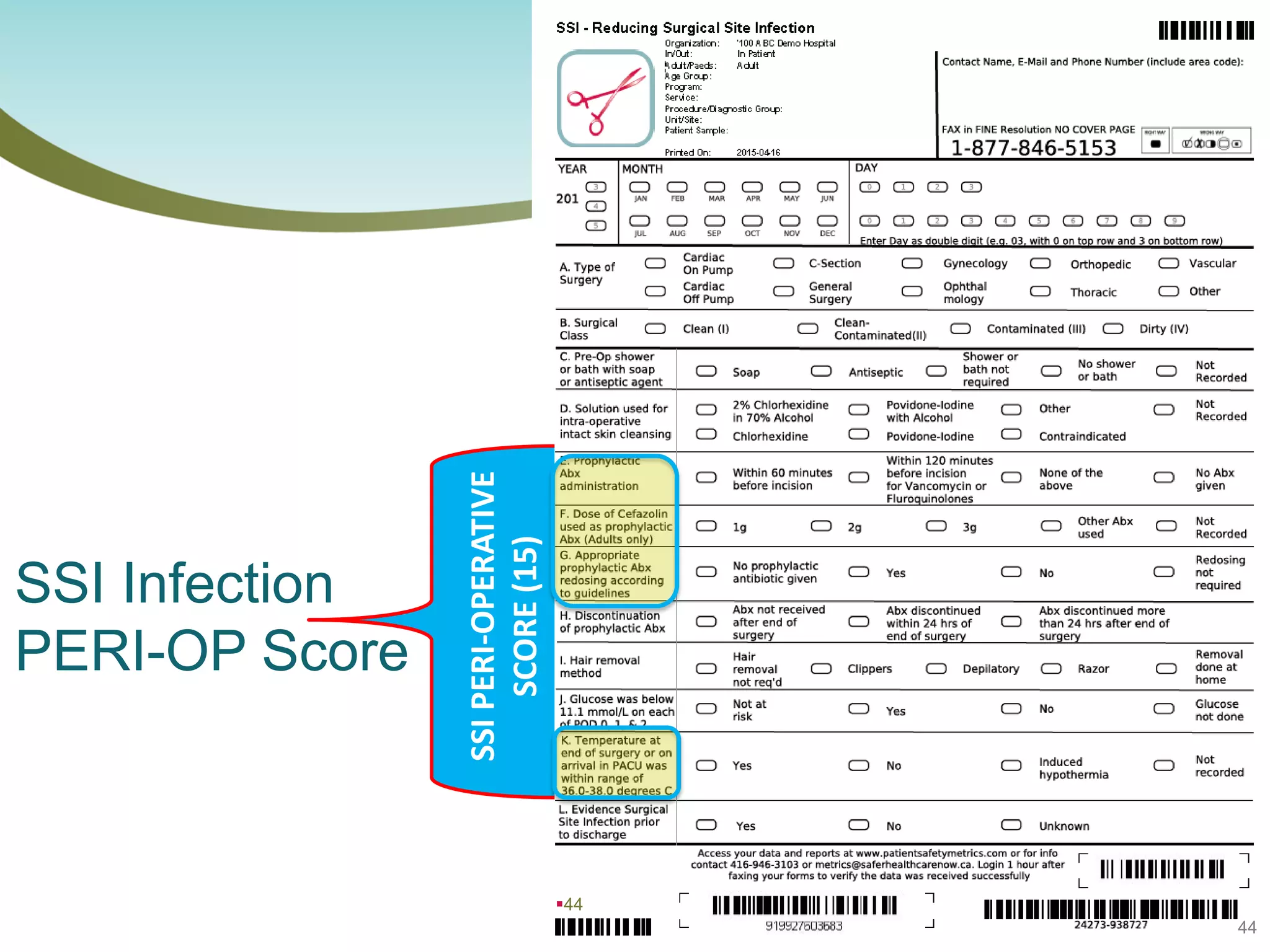
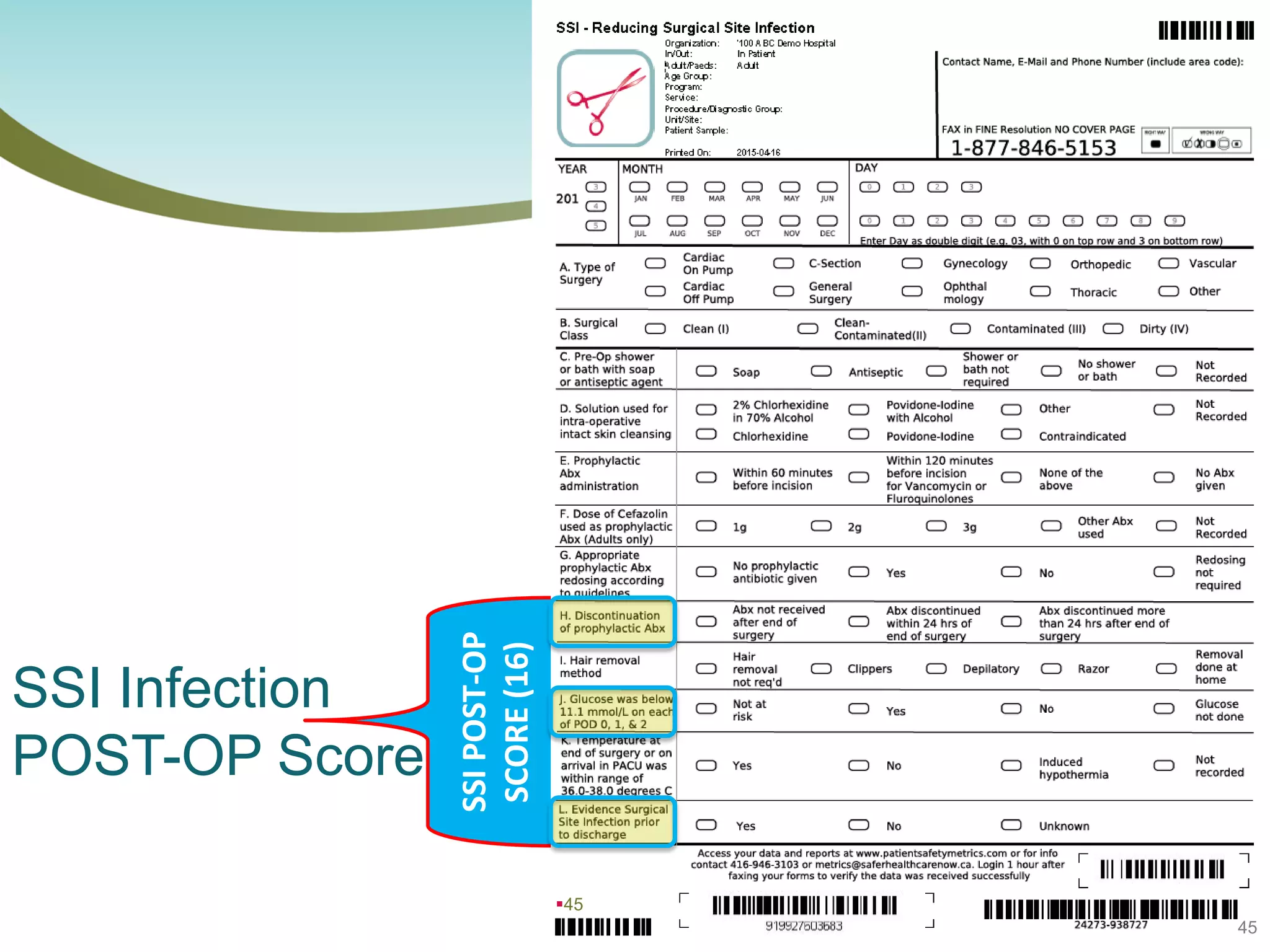
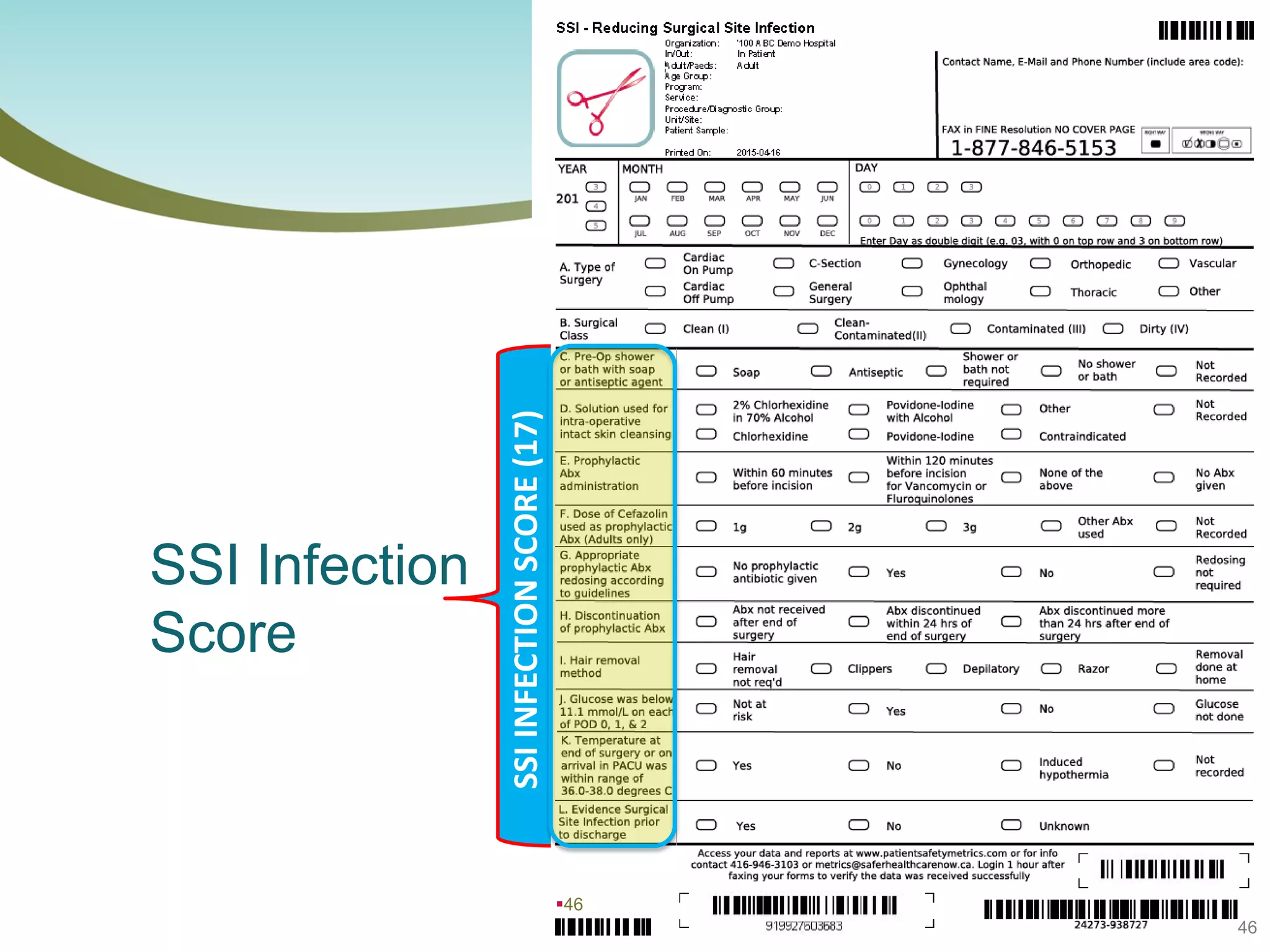
The document provides an introduction to the Surgical Site Infection (SSI) Audit Tool. It describes the objectives of the tool which are to retrospectively review patient charts and measure processes to help drive continuous improvement in preventing SSIs. The tool measures adherence to best practices across pre-operative, peri-operative, and post-operative periods using a series of yes/no questions. Hospitals are encouraged to begin using the tool to collect monthly SSI data and track their progress towards reduction goals. Support is available from Central Measurement Team and SSI experts to help sites implement and utilize the audit tool.