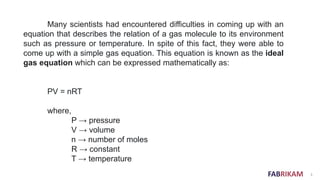
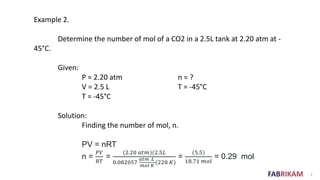
The document discusses respiration and how it works. As we inhale, the diaphragm moves down which reduces pressure in the lungs and causes air to rush in. As we exhale, the diaphragm pushes up increasing pressure in the lungs and forcing the air out. Breathing involves small pressure differences between the inside and outside of the lungs. A normal breath contains about 0.21 moles of air, which is enough to sustain life.