More Related Content
PPTX
Hydrocarbons_10th_Class_111Version2.pptx PDF
Hydrocarbons Alkane, Alkene and Alkyne.pdf PPT
PPTX
CHAPTER 2 INTRODUCTION TO CARBON COMPOUND PPTX
Carbon and Its compounds. [ Class- X ] PPTX
hydrocarbon class 11 ppt.pptx PPTX
Types_of_Hydrocarbons_and_Nomenclature_Class_11.pptx PPTX
Hydrocarbons_Presentation.pptx IN DETAILED BY ME Similar to Hydrocarbons_10th_Class_ewwwVersion3.pptx
PPTX
hydrocarbons-introduction,alkanes and alkenes.pptx PPTX
Introduction to Organic Chemistry.pptx PPTX
Chapter 20.3 : Saturated and Unsaturated Hydrocarbons PPTX
Notes- Organic day 2.pptx PDF
DOC
Innovative teaching manual PPT
307-Alkanes.ppt-Pharmaceutical Organic Chemistry PPTX
HYDROCARBONS AND FUNCTIONAL GROUPS.pptx gr PPTX
lecture-3 updated all about hydrocarbons.pptx PPTX
PPTX
Alkynes and hydrogenation of alkynes PDF
ALKENES and it Functional Groups Compund PPTX
Hydrocarbons_Lecture_Ms_Sameera_Sajjad.pptx PPT
Hydrocarbons_Lecture_Ms_Sameera_Sajjad.ppt PPTX
PPTX
Alkane alkene and alkynes PDF
Chemistry ail shashank [hydrocarbons] PPT
P O S T L A B Biochem D L S L PPTX
PPTX
Recently uploaded
PPTX
English 8 Q3 Wk6.pptx prewriting opinion editorial article PPTX
PixelX : Think Design Experience powerpoint presentation PDF
Enhancing Accessibility and Inclusivity: The TU Dublin Leaving Certificate St... PDF
Homebound (2025): A Critical Analysis of Social Realism, Systemic Apathy, and... PPTX
From AI curious to AI conversant – a year of experimentation at TCD Library. ... PPTX
GRADE-1-Q3-MATH-WEEK-6.pptx_202633333333 PPTX
Chapter No. 5 Anti- Hypertensive Pharmacognosy-2.pptx PPTX
LEARNING PART 1. SHILPA HOTAKAR PSYCHOLOGY NOTES pptx PPTX
Inter School Quiz Competition Final.pptx PPTX
Conduction System of the Heart, The Cardiac Cycle, The Cardiac Output, ECG.pptx PDF
Visualising Library Insights: Power BI Dashboard for Data-Driven Decision Mak... PPTX
LEARNING PART 2.SHILPA HOTAKAR PSYCHOLOGY NOTES pptx PDF
Fever & Fragment Uncovering the Plague in The Waste Land PPTX
Bookish Bats: a unique sustainable delivery service in the Russell Library. A... PDF
Old Historicism vs New Historicism Slides PDF
Pharmaceutical Biotechnology unitI Notes PDF
‘You can do anything, but not everything’: Using UCC’s Service Principles to ... PDF
A predictive coding framework for rapid neural dynamics during sentence-level... PPTX
AI Literacy at UCD Library. Dr Marta Bustillo & Sandra Dunkin, University Col... PPTX
Cardiovascular System or The Details of Heart.pptx Hydrocarbons_10th_Class_ewwwVersion3.pptx
- 1.
- 2.
Introduction
• Hydrocarbons: compoundsmade up of Carbon (C) and Hydrogen (H).
• Main categories:
• • Alkanes → Saturated (single bonds).
• • Alkenes → Unsaturated (double bond).
• • Alkynes → Unsaturated (triple bond).
- 3.
Alkanes (CnH2n+2)
• GeneralFormula: CnH2n+2
• Tetrahedral geometry around carbon atoms.
• Examples: CH4 (Methane), C2H6 (Ethane), C3H8 (Propane).
• Nomenclature: root name + 'ane'.
• Reactions:
• • Complete combustion: CH4 + 2O2 → CO2 + 2H2O
• • Substitution: CH4 + Cl2 → CH3Cl + HCl (UV light)
- 4.
Alkenes (CnH2n)
• GeneralFormula: CnH2n
• Planar structure around double bond carbon atoms.
• Examples: C2H4 (Ethene), C3H6 (Propene).
• Nomenclature: root name + 'ene'.
• Reactions:
• • Combustion: C2H4 + 3O2 → 2CO2 + 2H2O
• • Addition: C2H4 + Br2 → C2H4Br2 (Bromination).
• • Polymerization: n(C2H4) → (C2H4)n (Polyethene).
- 5.
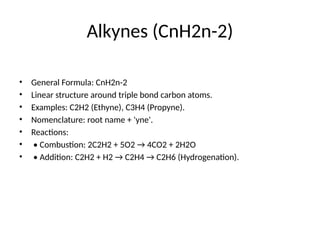
Alkynes (CnH2n-2)
• GeneralFormula: CnH2n-2
• Linear structure around triple bond carbon atoms.
• Examples: C2H2 (Ethyne), C3H4 (Propyne).
• Nomenclature: root name + 'yne'.
• Reactions:
• • Combustion: 2C2H2 + 5O2 → 4CO2 + 2H2O
• • Addition: C2H2 + H2 → C2H4 → C2H6 (Hydrogenation).
- 6.
Formation of Hydrocarbons
•Alkanes: Hydrogenation of alkenes/alkynes.
• Alkenes: Dehydration of alcohols, cracking of alkanes.
• Alkynes: Dehydrohalogenation of dihalides.
• Natural sources: Petroleum, Natural Gas, Coal.
- 7.
Comparison of Hydrocarbons
•Alkanes → Single bond, least reactive, undergo substitution.
• Alkenes → Double bond, reactive, undergo addition reactions.
• Alkynes → Triple bond, highly reactive, undergo addition reactions.
- 8.
Applications
• Alkanes: Fuels(methane, propane, butane).
• Alkenes: Plastics (polyethene, PVC), solvents, alcohol synthesis.
• Alkynes: Welding (oxy-acetylene torch), organic synthesis.
- 9.
Summary
• Hydrocarbons areessential organic compounds.
• Different types depend on C–C bond (single, double, triple).
• Alkanes (CnH2n+2) → Saturated hydrocarbons.
• Alkenes (CnH2n) → Unsaturated, double bond.
• Alkynes (CnH2n-2) → Unsaturated, triple bond.