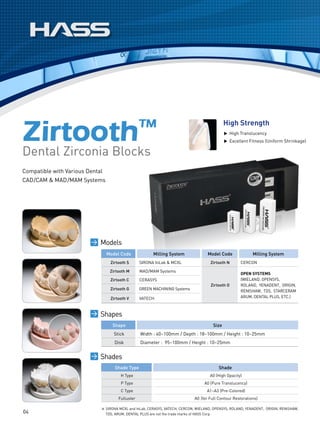
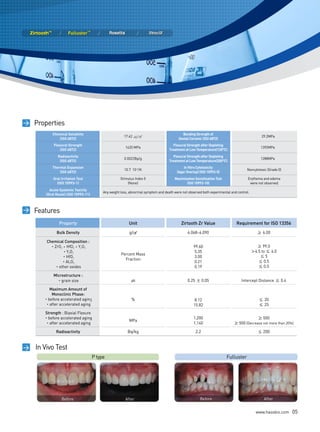
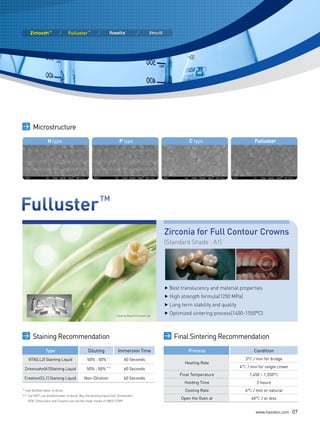
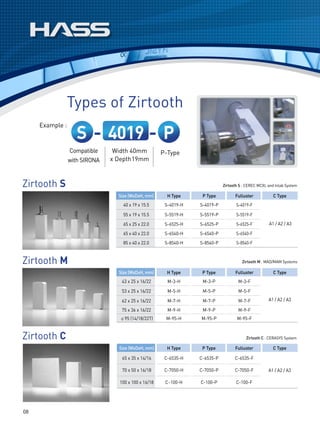
This document discusses all-ceramic materials for all-ceramic dental restorations. It describes various zirconia blocks and ingots compatible with different CAD/CAM and pressing systems, as well as glass ceramic blocks. The zirconia materials demonstrate high strength, excellent biocompatibility and clinical durability. Glass ceramic blocks are also presented with flexural strengths up to 350 MPa. The document emphasizes contributing to better health, beauty, and bio-materials technology through reasonable prices, satisfactory esthetics, sufficient strength, and bio-compatibility of the ceramic materials.




![Sizes
Blocks[BM / SM] Ingots [SP]
Code Dimensions (mm) Code Dimensions (mm)
C8 8 x 8 x 15
R10 φ12.7 x 10
C10 8 x 10 x 15
C12 10 x 12 x 15
R20 φ12.7 x 20
C14 12 x 14 x 18
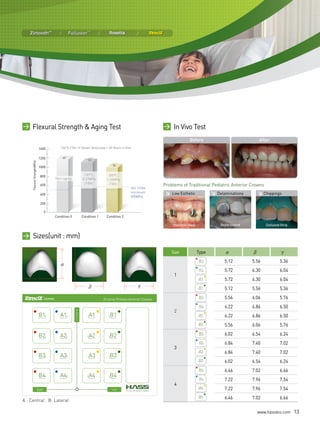
Test Results & Specifications of BM Series
| teSt ReSULtS |
• Chemical Solubility (ISO6872) : 46.67 ㎍/㎠
• Flexural Strength (ISO6872, Biaxial) : 130MPa
• Radioactivity (ISO6872) : 0.000248Bq/g
• Linear Thermal Expansion (ISO6872) : 9.73×10-6/K
• Glass Transition Temperature (ISO6872) : 626.55℃
• Cytotoxicity Test (ISO10993-5) : Noncytotoxic
• Acute Systemic Toxicity Test (ISO10993-11) : Do not show any systemic toxicity potential.
• Oral Mucosa Irritation Test (ISO10993-10) : Do not possess any oral mucosa irritation potential.
• Delayed Type Hypersensitivity Test (ISO10993-10) : Do not possess any delayed hypersensitivity.
| SpeCIFICAtIOnS |
property Unit Value Standard
Bulk Density g/㎤ 2.0~2.4 N/A
Chemical Composition
• SiO
2 65 > 60
• Al O
2 3 15 < 20
%
• K O
2 13 > 10
• other oxide 17 < 20
Flexural Strength MPa 130±10 > 50MPa ISO 6872(Biaxial)
Chemical Solubility g/㎠ 46.67 < 100 ISO 6872
Tg C° 626.55 ISO 6872
Radioactivity ISO 6872
• 238U Bq/g 0.000248 < 1.0 238U
• 226Ra 0.01 < 0.2 226Ra
www.hassbio.com 11](https://image.slidesharecdn.com/pdf-13184693054011-phpapp01-111012203910-phpapp01/85/hassbio-11-320.jpg)