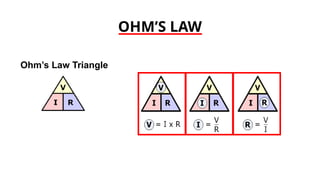
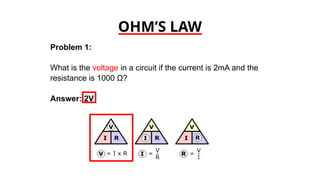
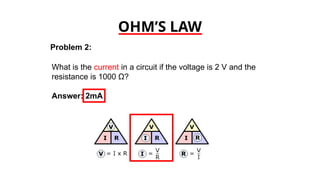
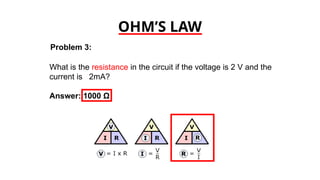
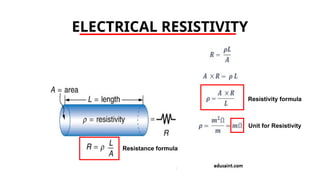
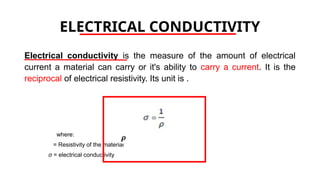
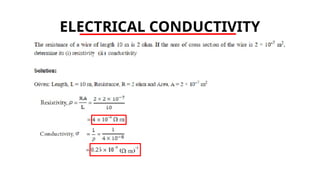
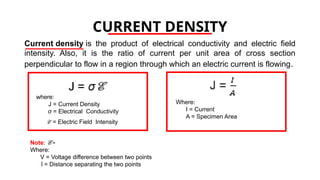
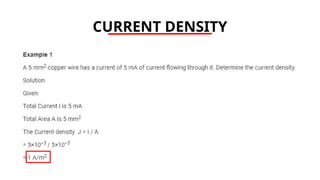
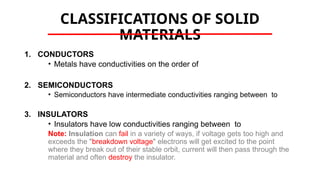
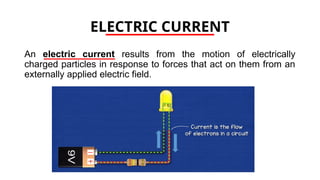
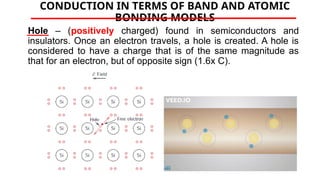
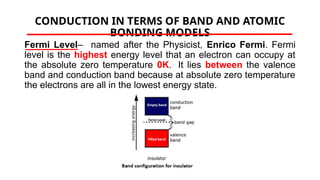
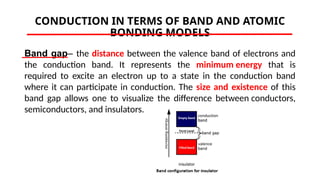
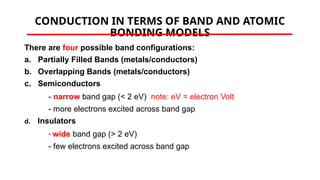
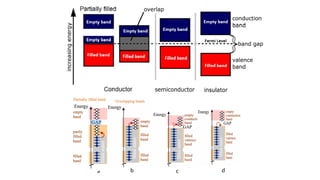
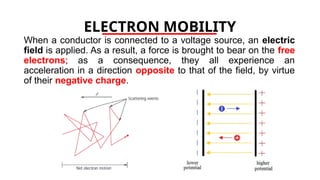
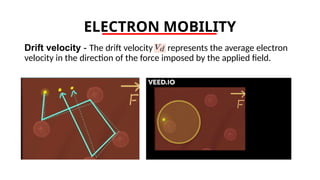
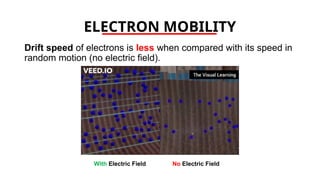
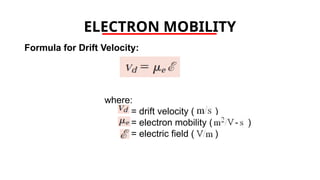
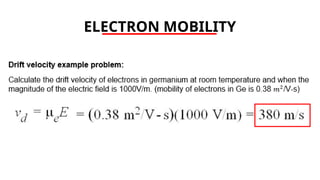
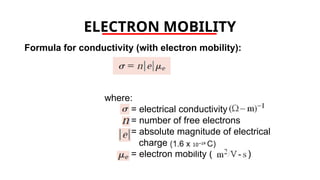
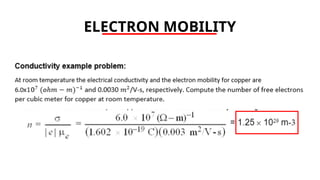
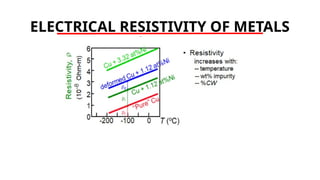
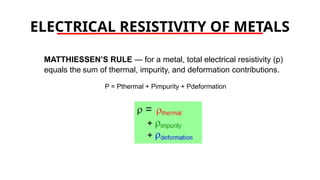
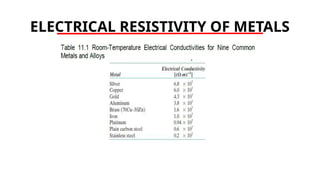
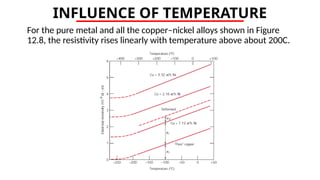
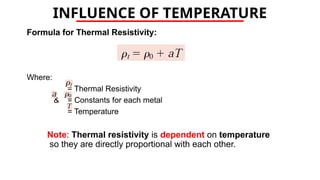
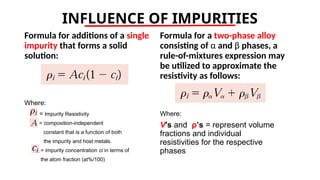
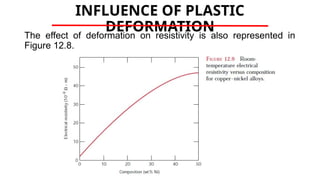
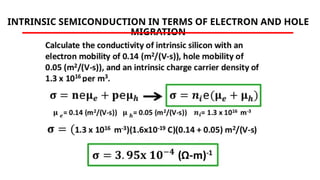
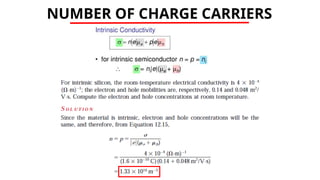
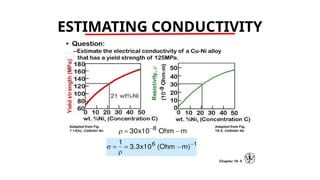
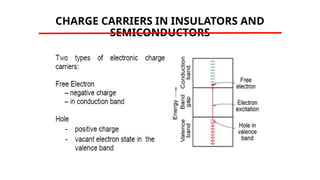
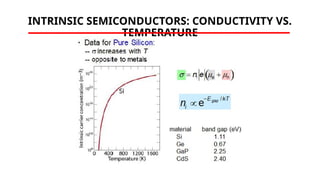
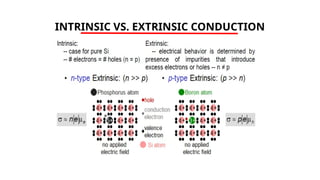
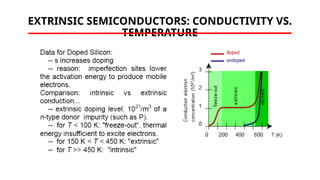
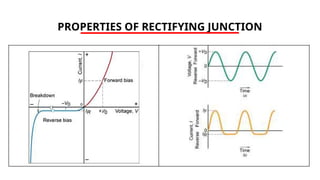
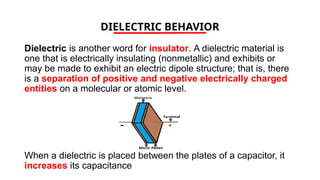
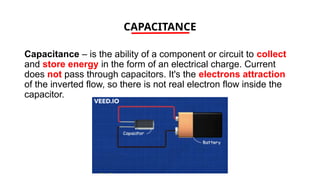
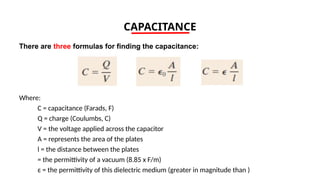
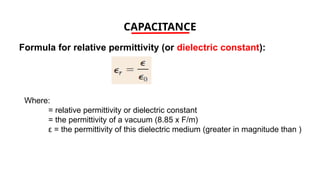
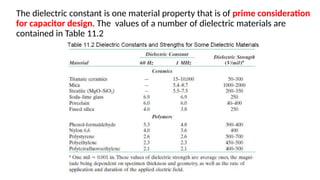
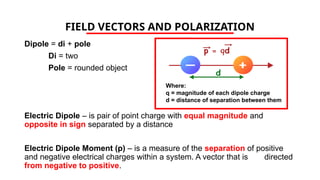
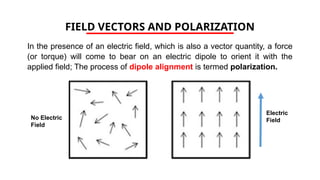
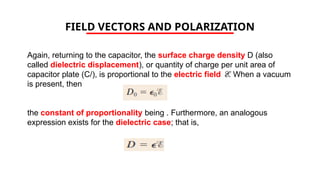
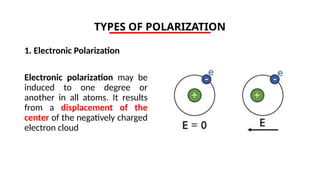
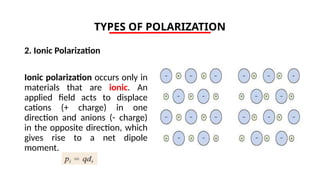
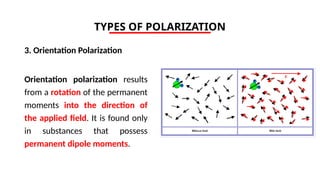
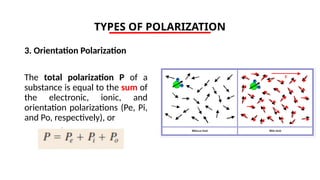
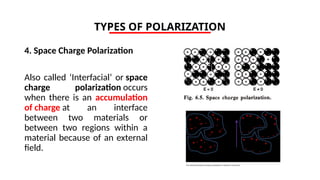
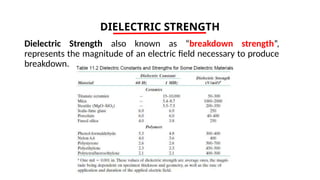
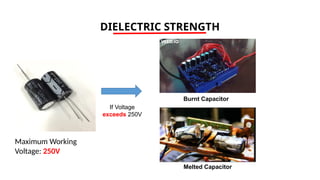
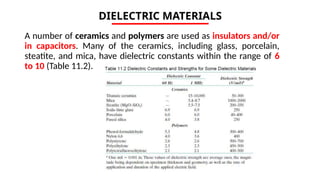
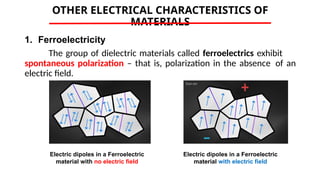
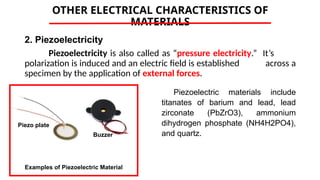
The document discusses electrical properties and dielectrical behavior, including Ohm's law, electrical resistivity, conductivity, and the behavior of semiconductors and dielectrics. It covers concepts such as current density, electron mobility, and types of semiconductors, along with their implications in electronic components like diodes and transistors. Additionally, it explains dielectric materials, their polarization mechanisms, and factors affecting dielectric strength and loss.