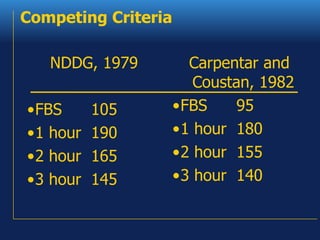
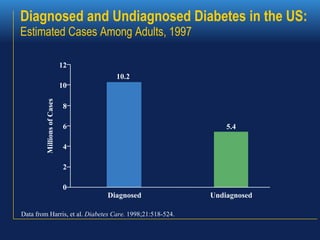
1. The document discusses the clinical management of diabetes during pregnancy, including screening, diagnosis, and treatment of gestational and pregestational diabetes.
2. It outlines the risks of hyperglycemia for both mother and fetus, including fetal macrosomia, complications during delivery, and long-term risks like childhood obesity.
3. The management of diabetes during pregnancy involves tight glycemic control through diet, glucose monitoring, and insulin when needed to improve outcomes for both mother and baby.