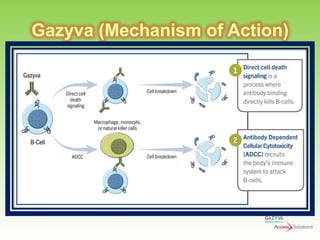
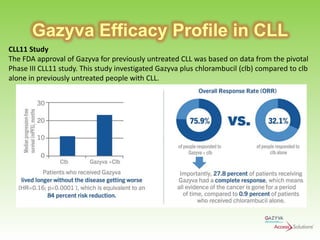
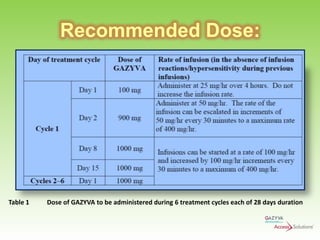
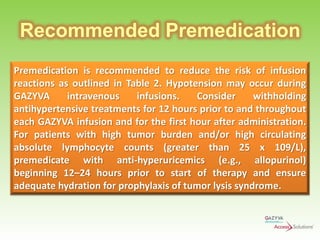
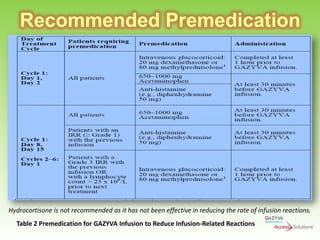
Gazyva (obinutuzumab) is a humanized anti-CD20 monoclonal antibody used for treating previously untreated chronic lymphocytic leukemia (CLL) in combination with chlorambucil chemotherapy. The drug targets CD20 on B-cells and has shown to improve survival without disease progression compared to chlorambucil alone in clinical trials. Administration requires careful premedication and monitoring due to potential severe infusion reactions.