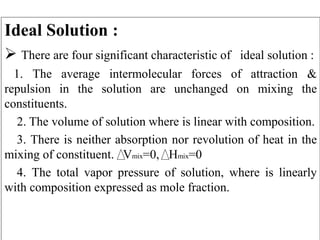
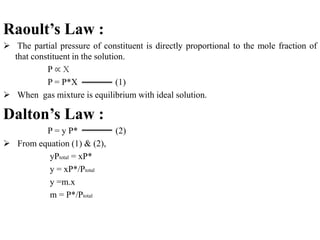
This document summarizes gas absorption and adsorption. It defines gas absorption as contacting a gas mixture with a liquid to dissolve one or more gas components. Requirements include mass transfer of a substance from gas to liquid. Equilibrium solubility of a gas in liquid increases with pressure and decreases with temperature. Selection of solvent depends on factors like gas solubility, solubility, cost, viscosity, and chemical stability. Ideal solutions follow Raoult's and Dalton's laws, while non-ideal solutions use Henry's law. Material balances are presented for one-component gas-liquid systems in absorption. Equilibrium curves are used to represent operating lines, which are typically non-linear.
![Semester : 5 [2051-CH]
Gas Absorption & Adsorption
Prepared by: Guided by:
Raut Krish(216470305008) Mr. K. S. Chavda
Patra Swarnlata(216470305013 )
Ambaliya Owaish(216470305014)](https://image.slidesharecdn.com/gasabsorptionadsorption-231030114122-933b271d/85/Gas-Absorption-Adsorption-pptx-1-320.jpg)