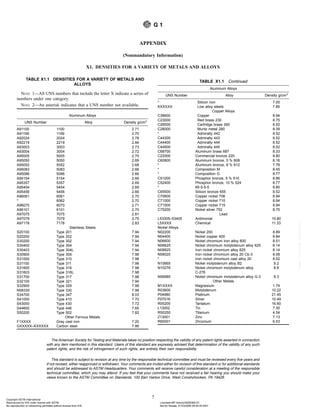
This document outlines standard practices for preparing, cleaning, and evaluating corrosion test specimens. It discusses procedures for preparing specimens prior to testing, including marking, surface finishing, and measuring. After testing, it recommends methods for removing corrosion products without significant base metal removal, including mechanical, chemical, and electrolytic cleaning. It provides tables of chemical and electrolytic cleaning procedures. Finally, it describes how to assess corrosion damage by calculating corrosion rates using measurements of specimen mass loss, surface area, exposure time, and material density.