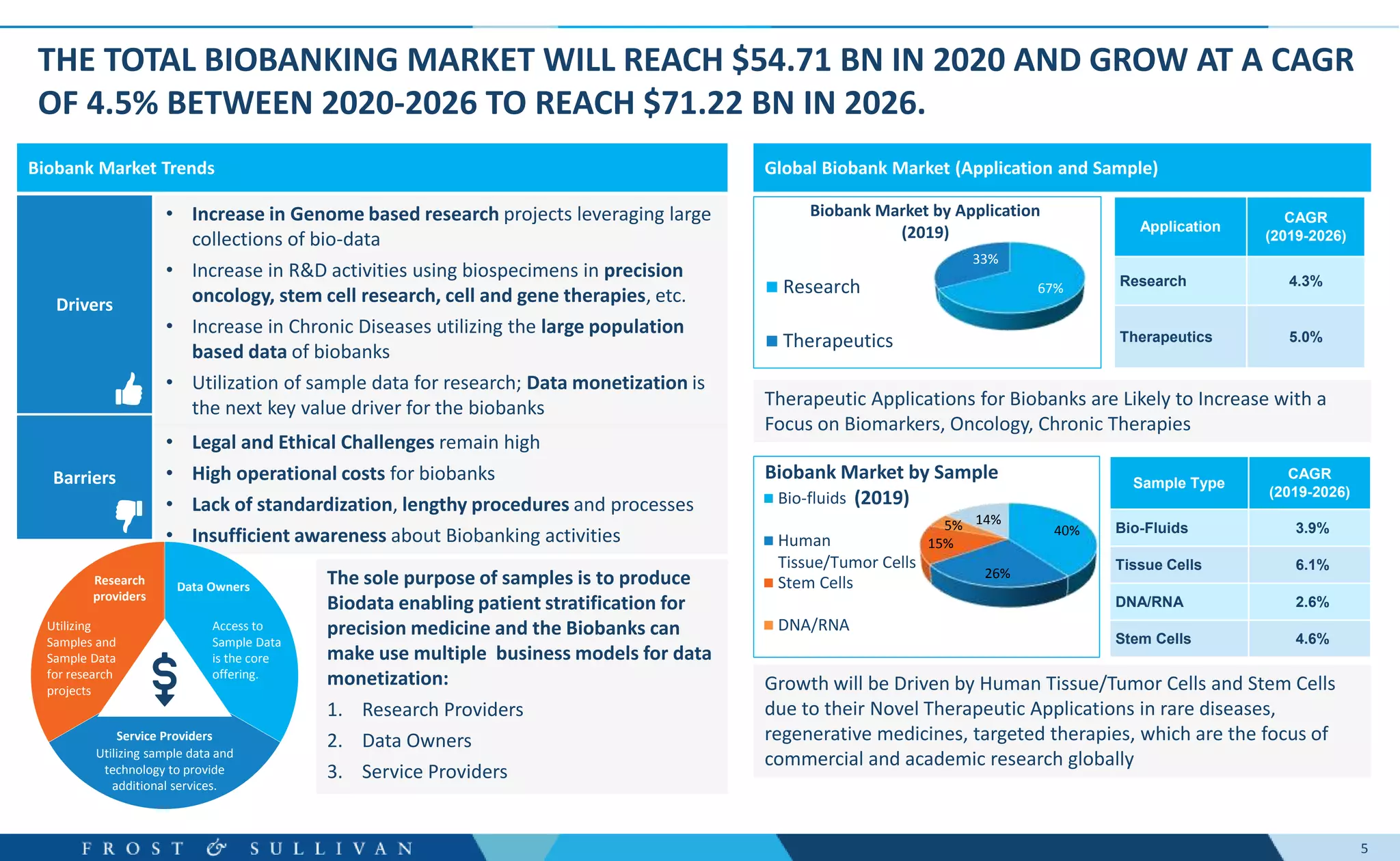
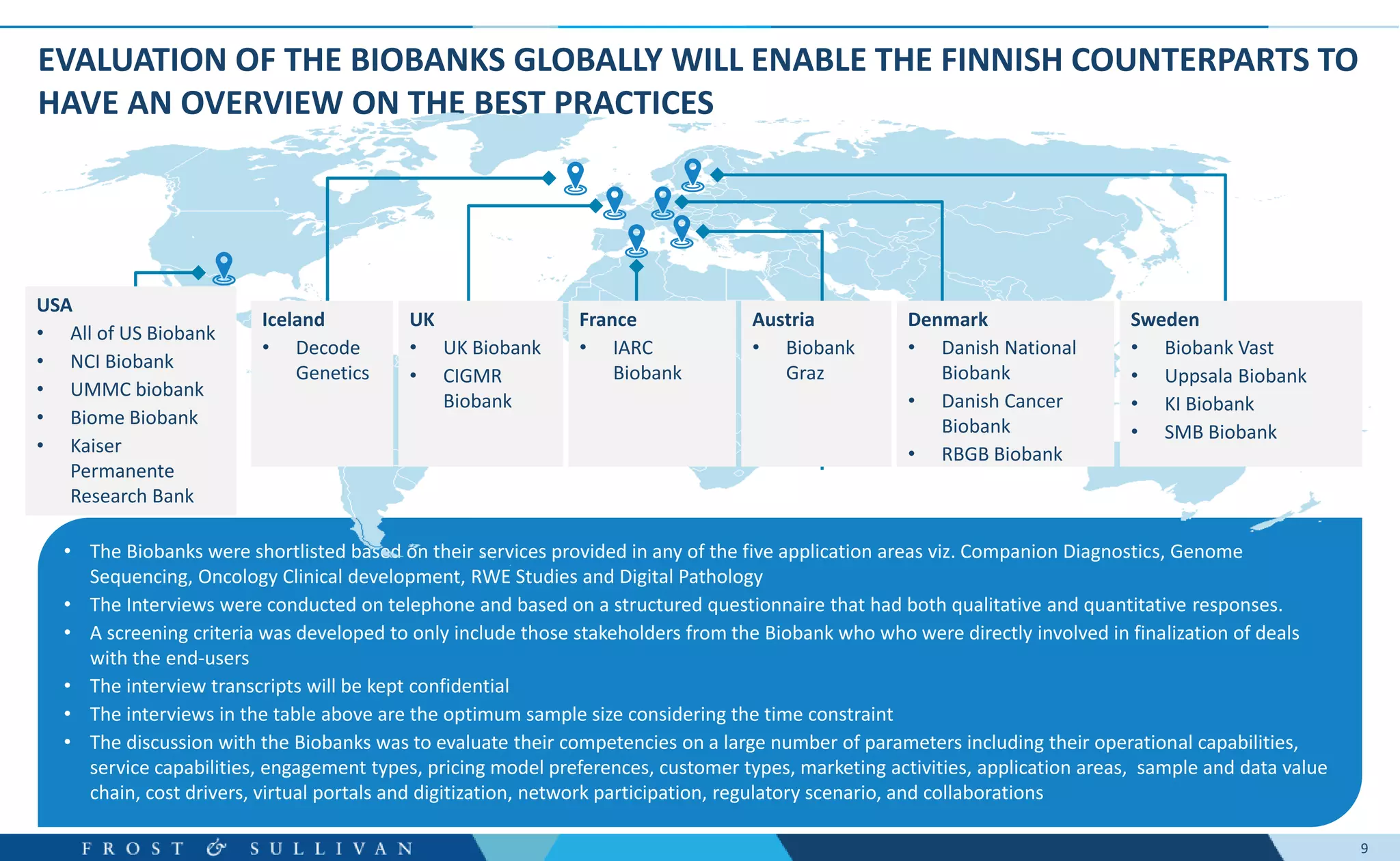
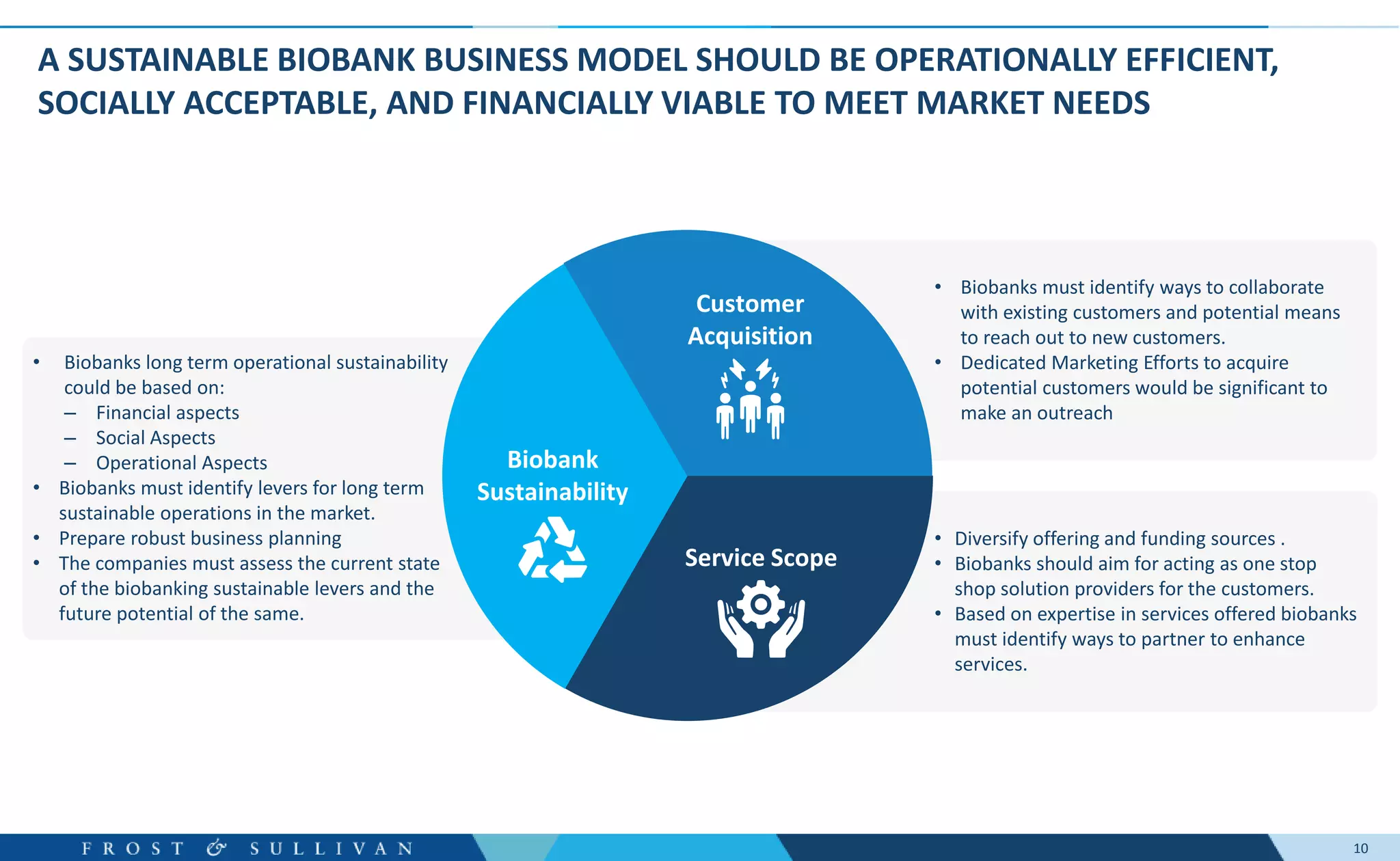
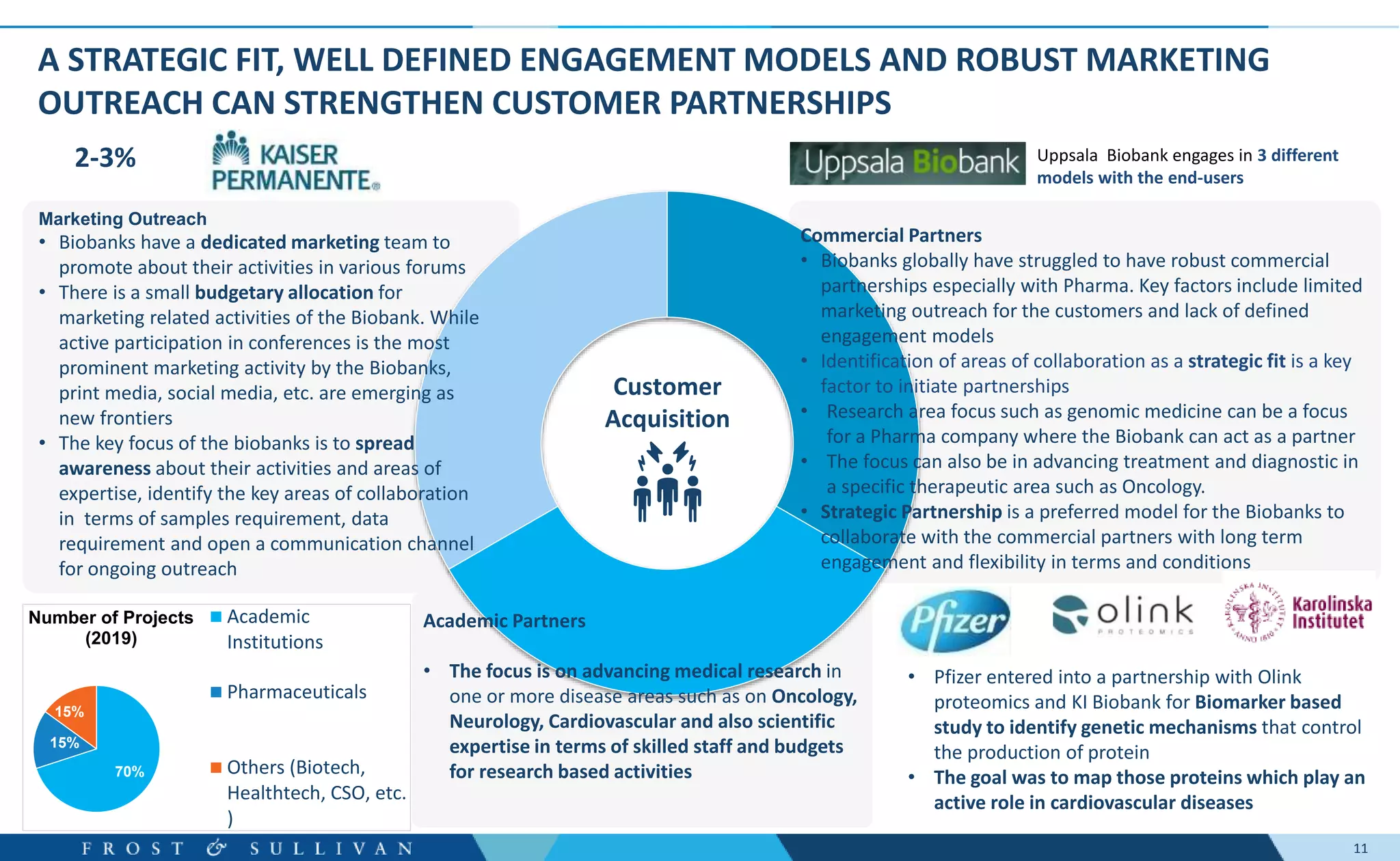
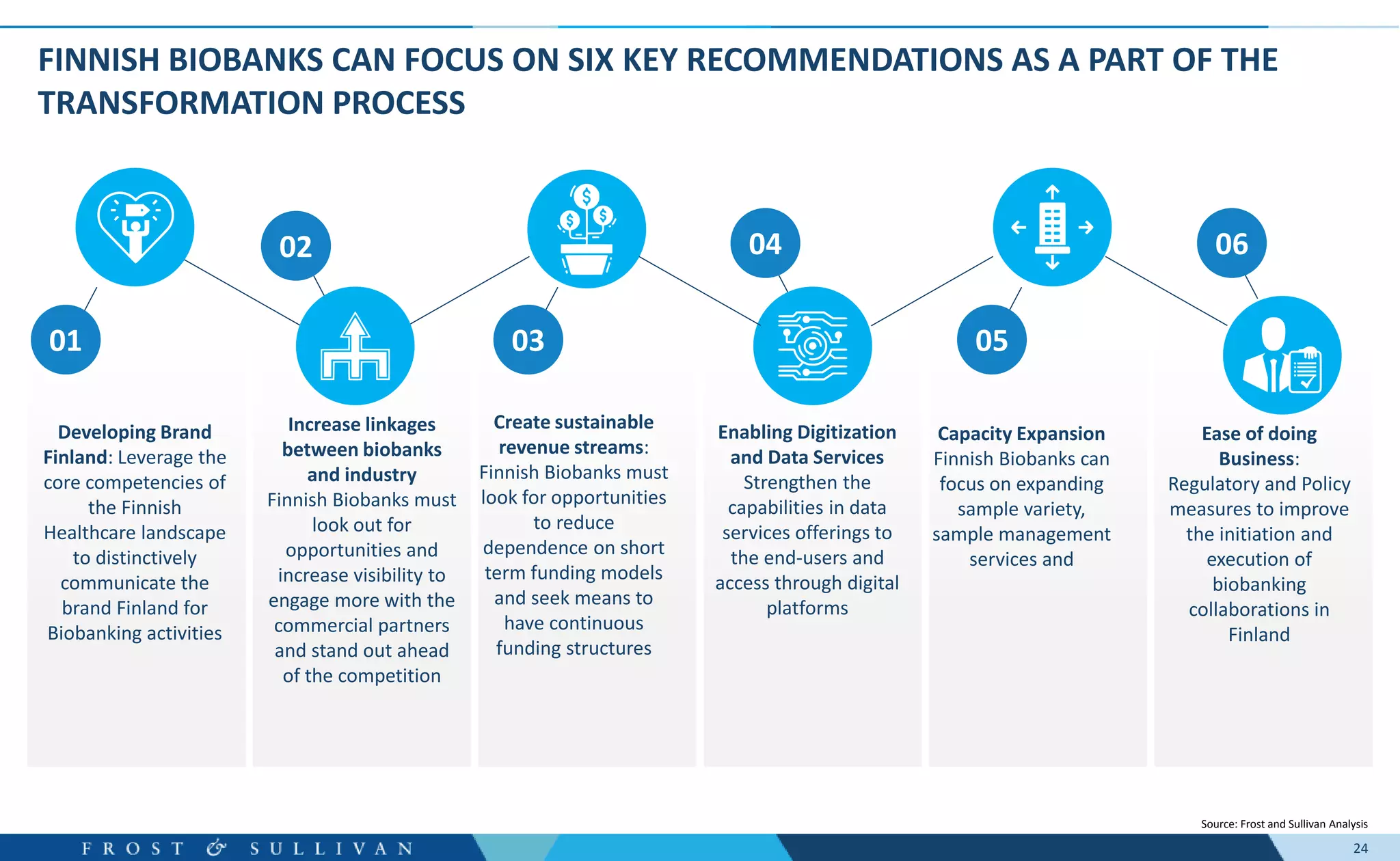
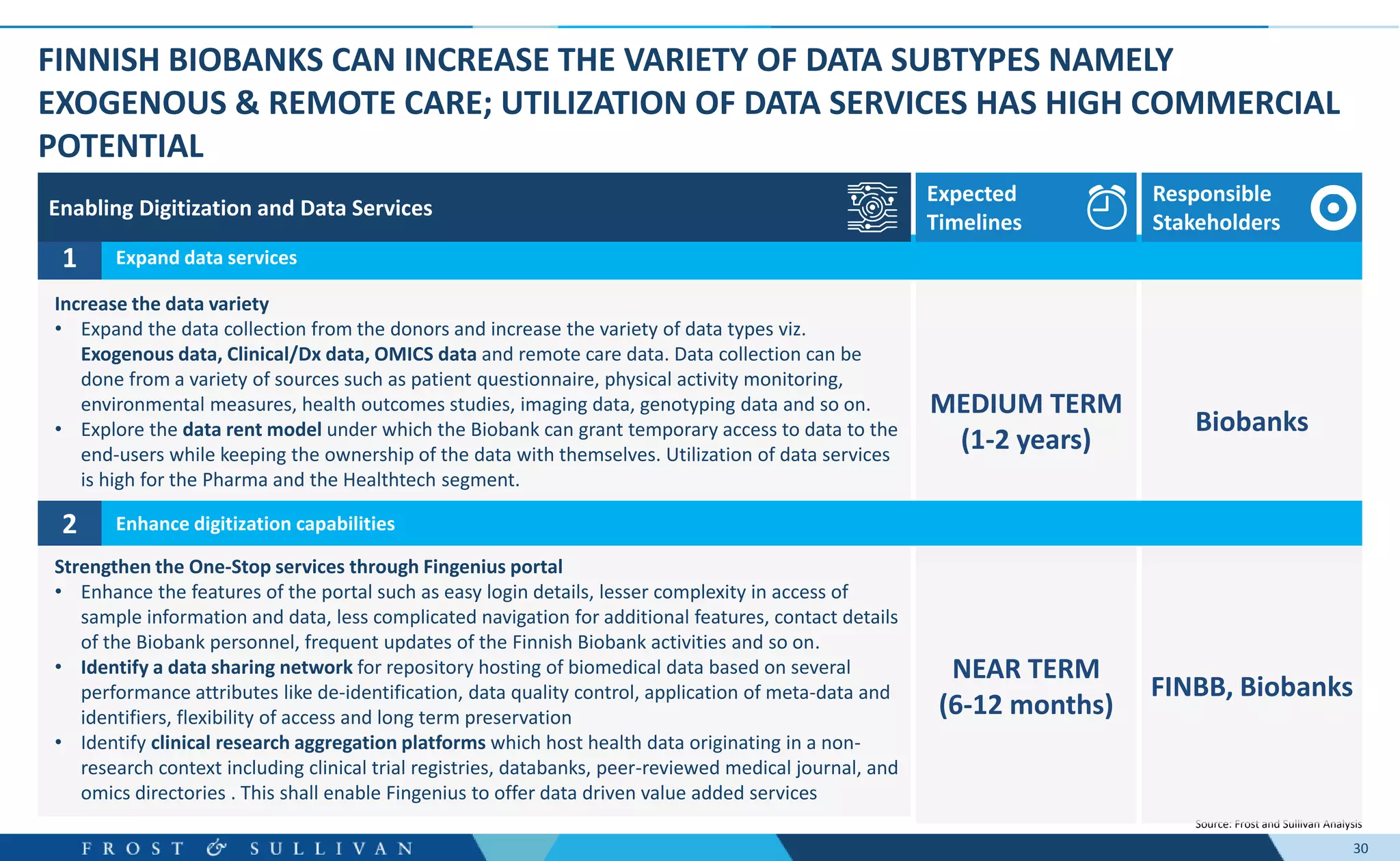
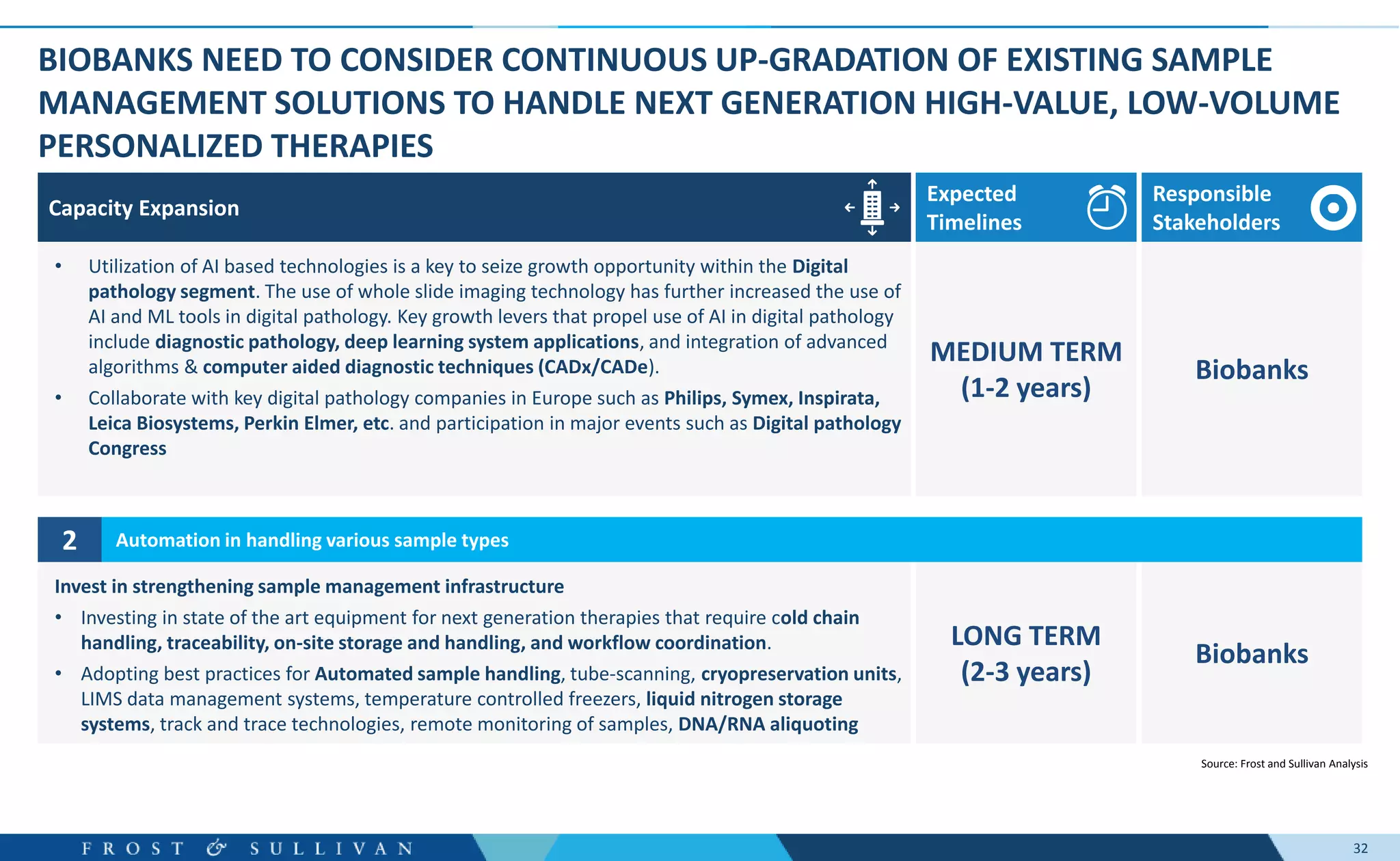
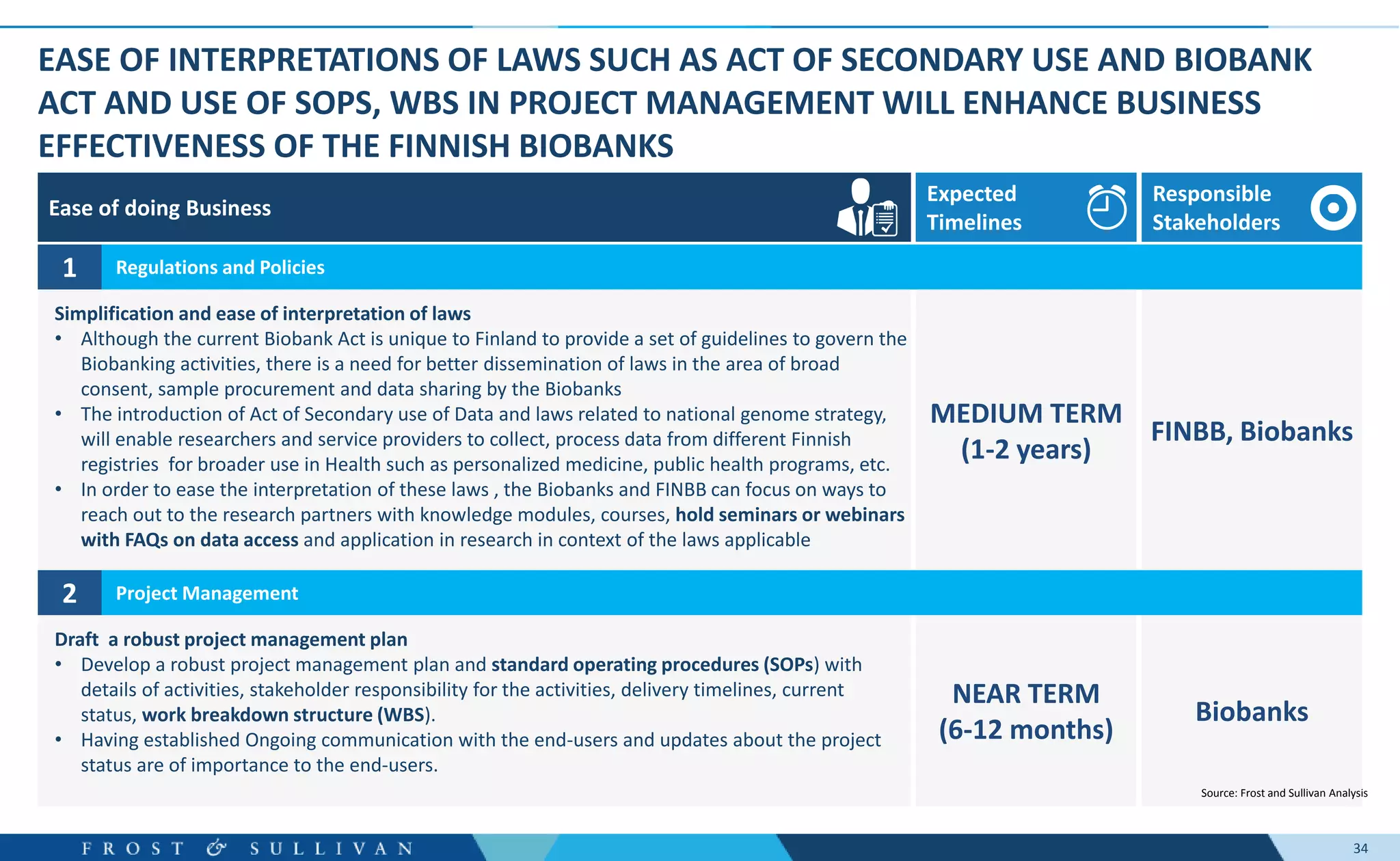
The document summarizes an assessment of growth opportunities for the Finnish bio-bank ecosystem. It conducted interviews with biobanks globally to understand best practices, as well as end-users to understand their needs. Key findings include:
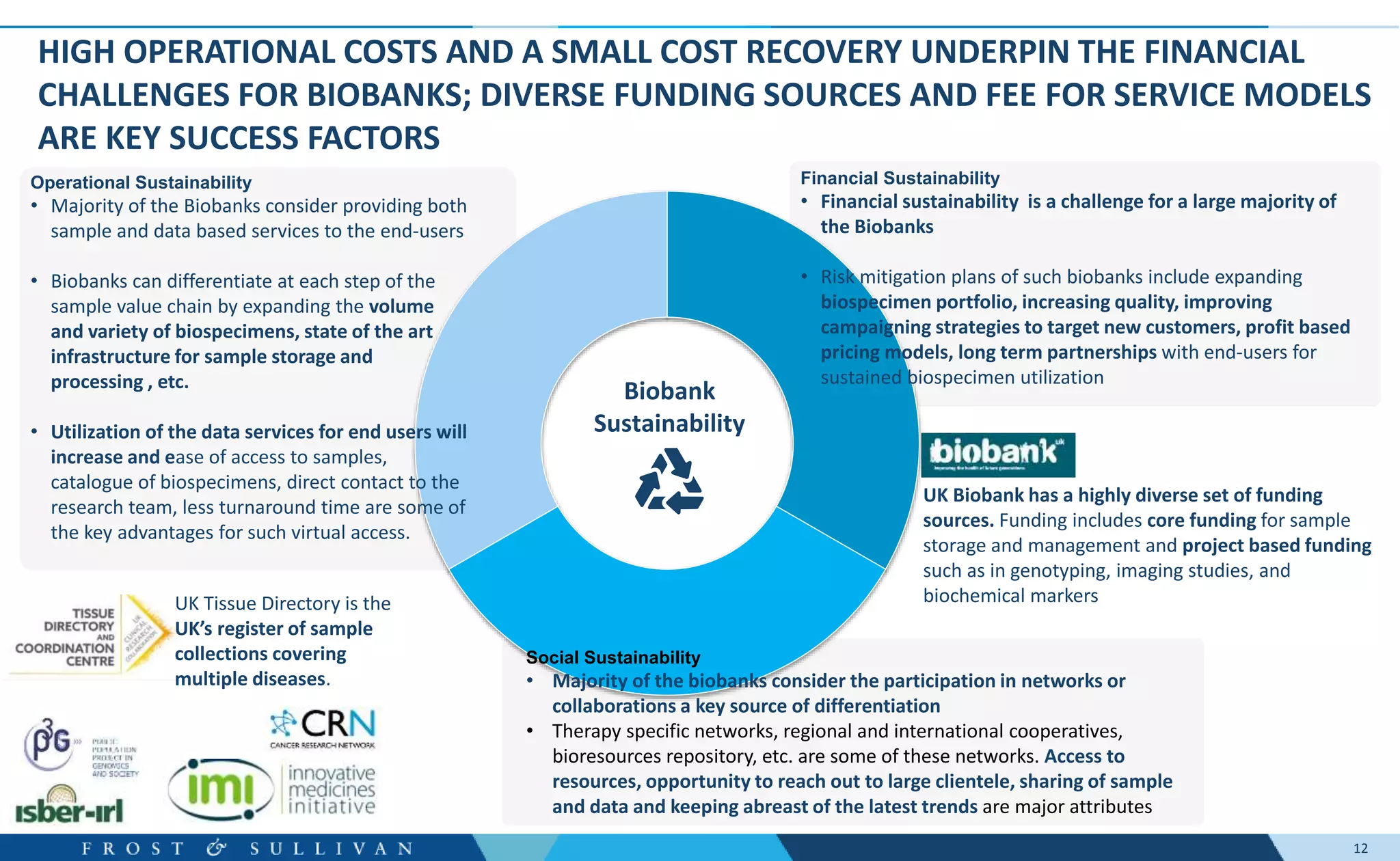
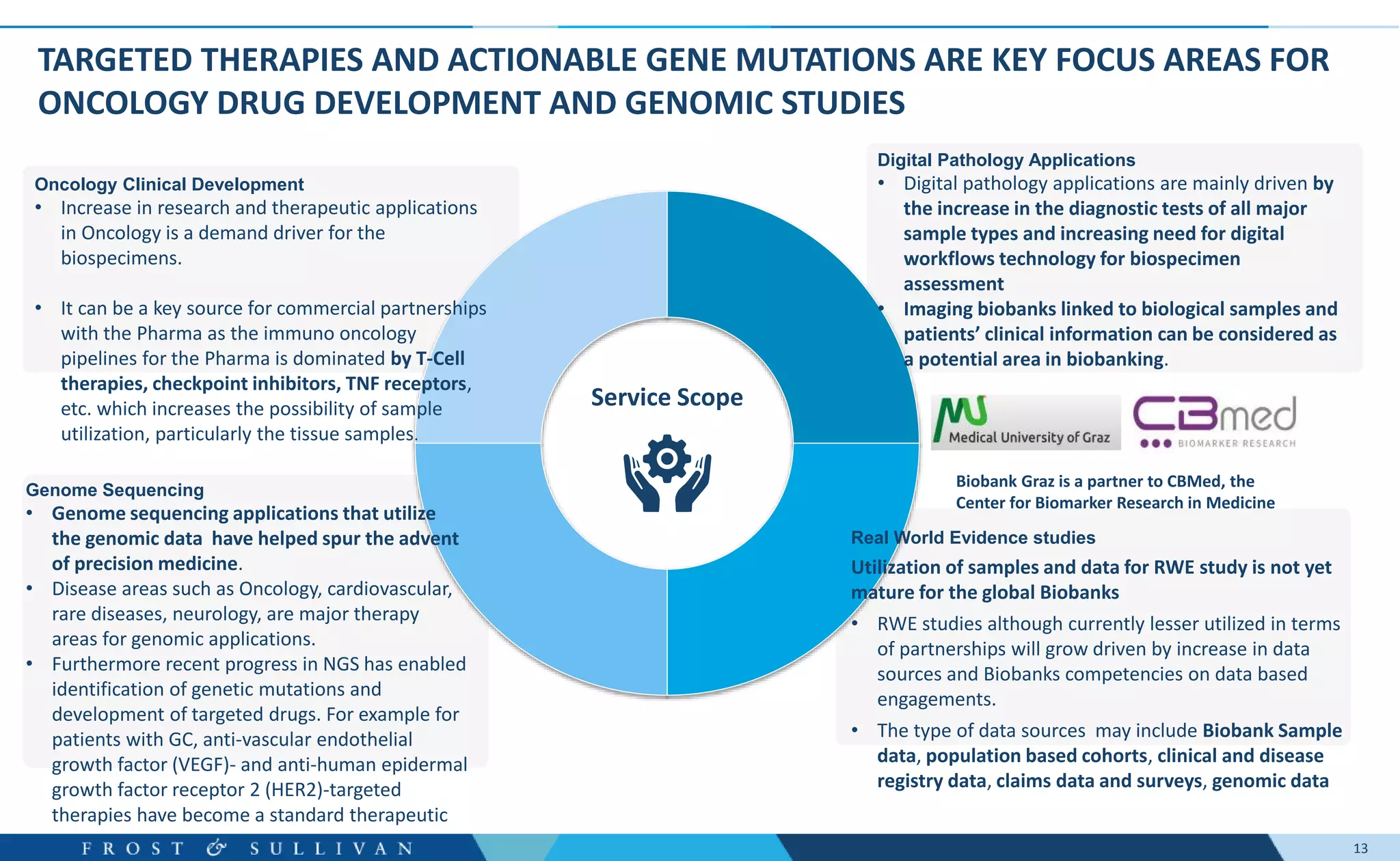
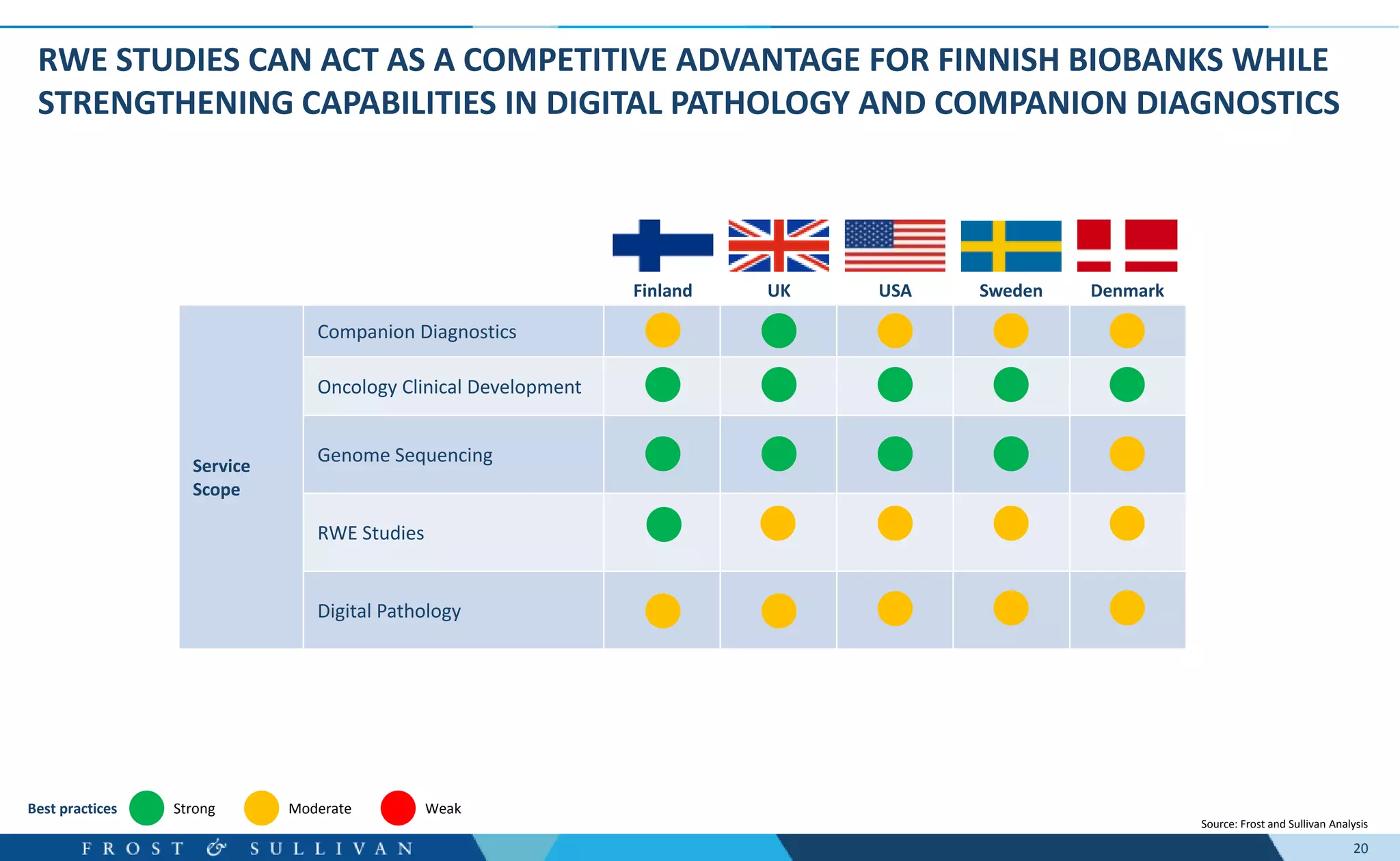
- Oncology clinical development, genome sequencing, and digital pathology were identified as major growth opportunities.
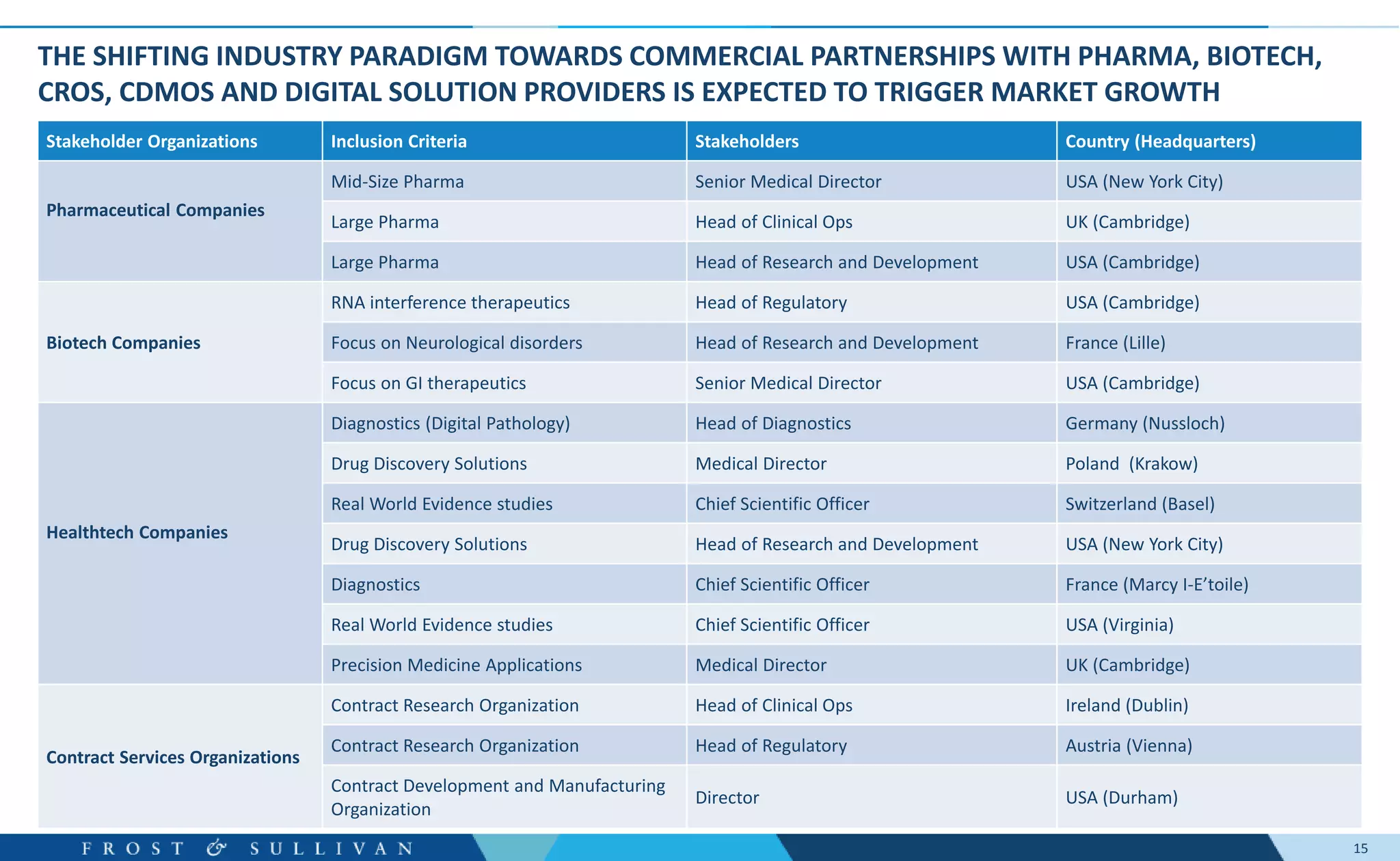
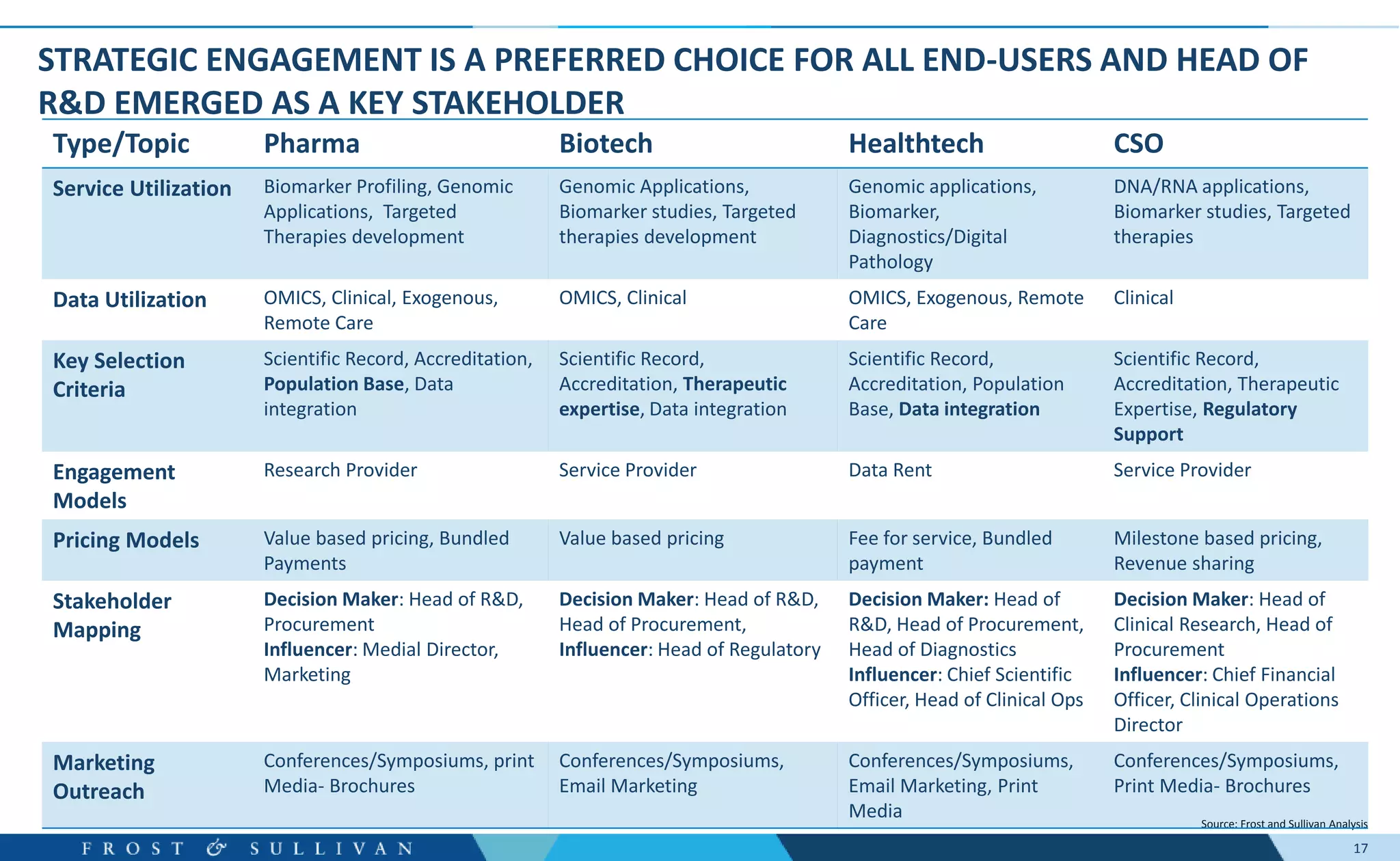
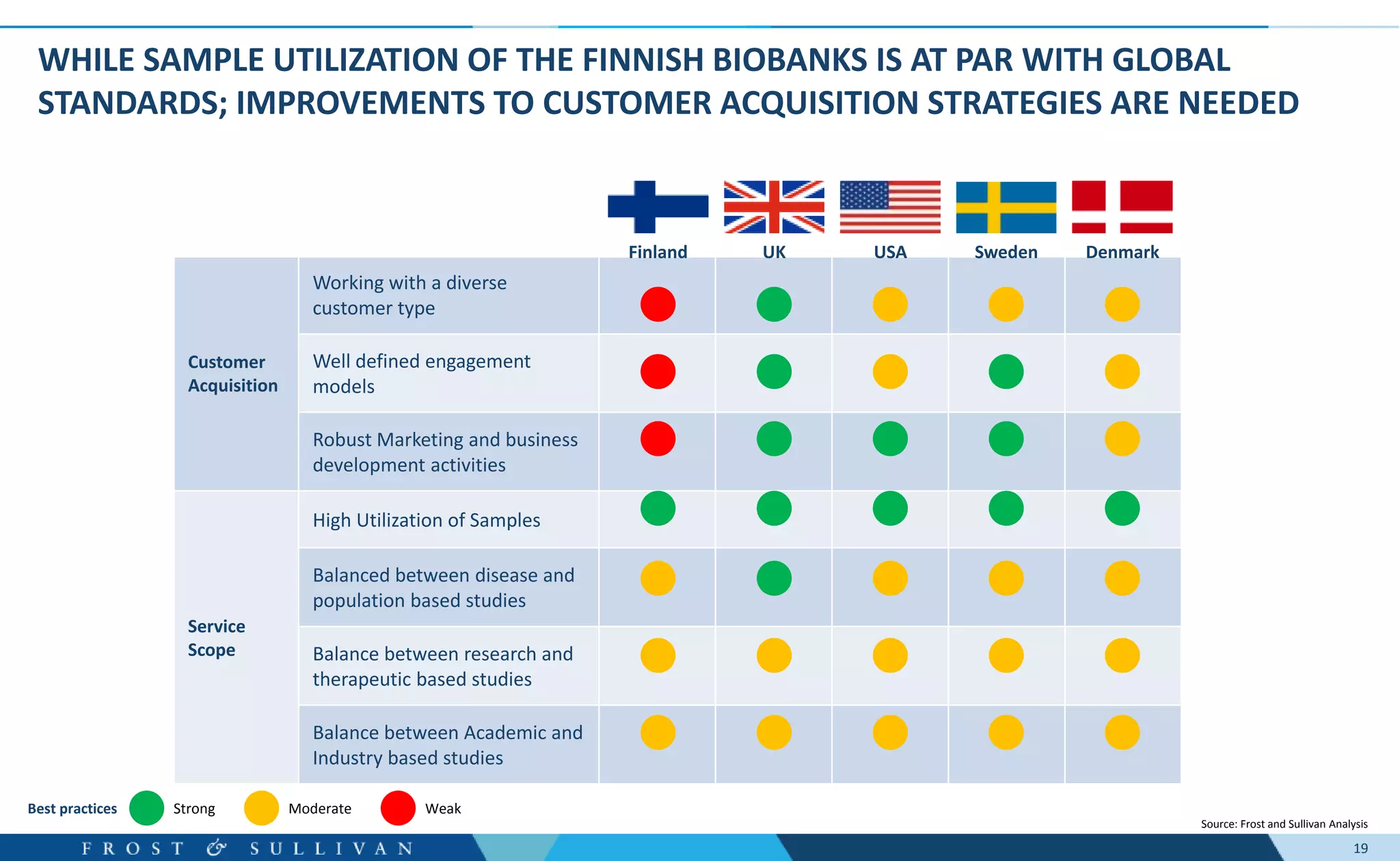
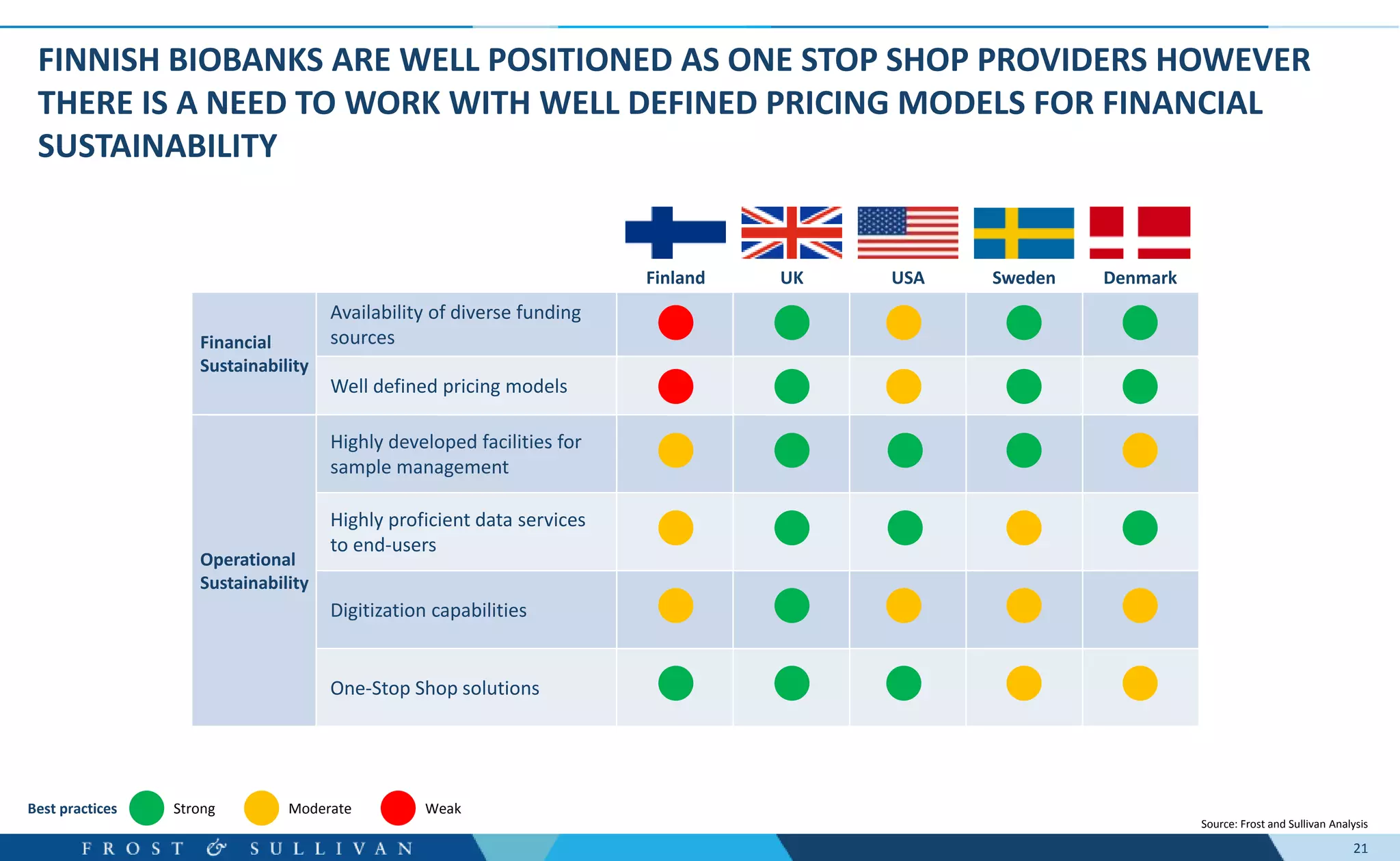
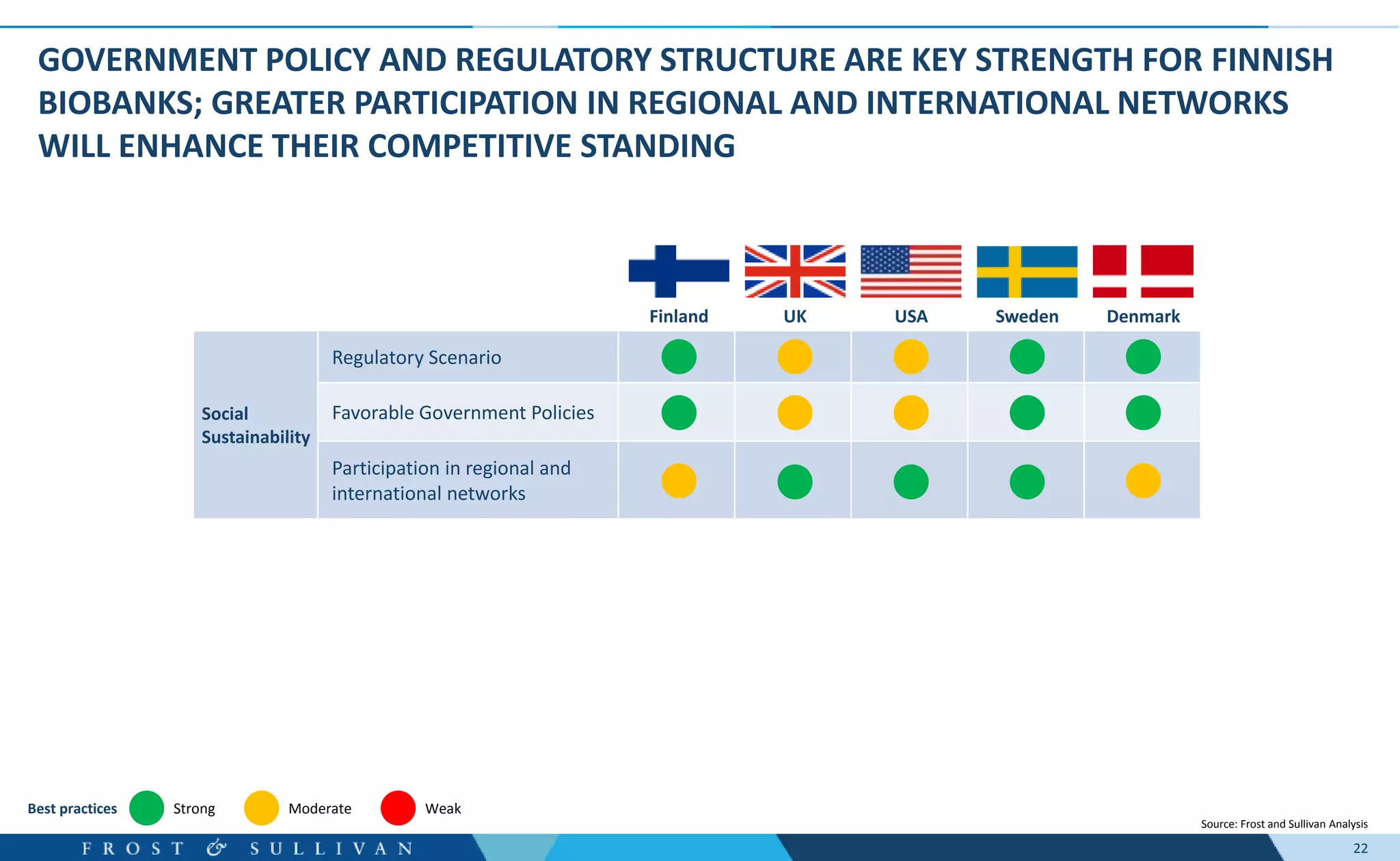
- Interviews found that marketing outreach, defined engagement models, and strategic partnerships are important for customer relationships.
- End-user interviews focused on service alignment, criteria for selecting biobanks, and partnership fit. Understanding end-user needs is critical for growth.