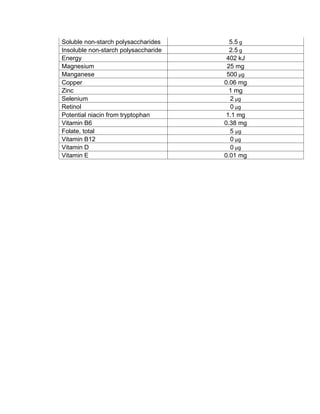
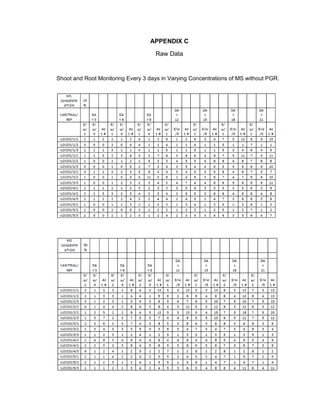
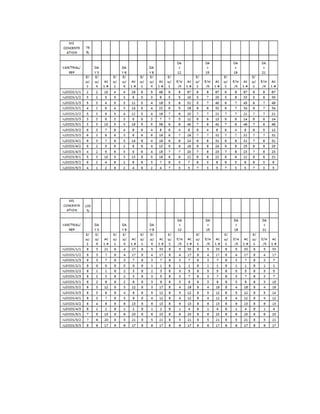
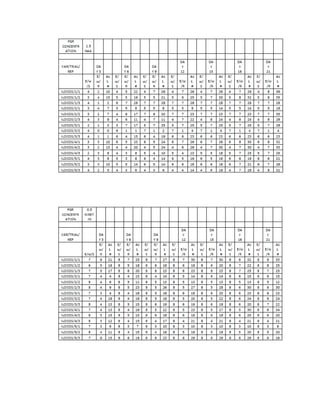
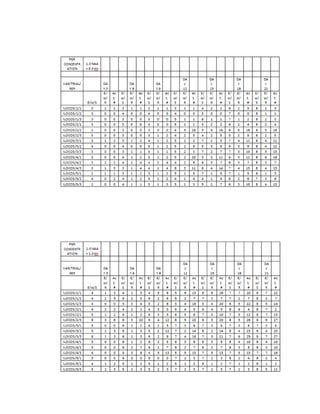
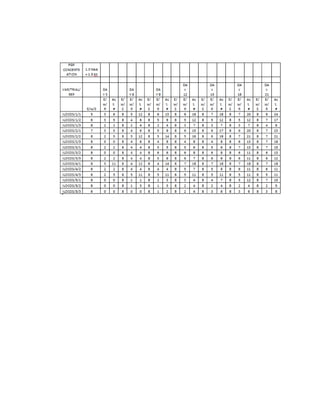
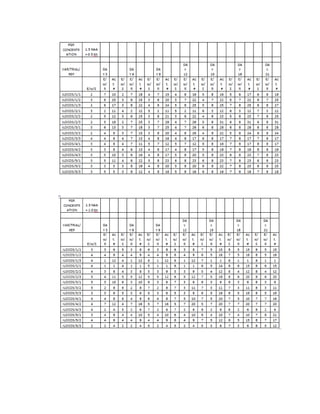
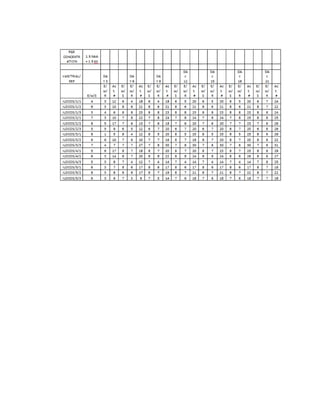
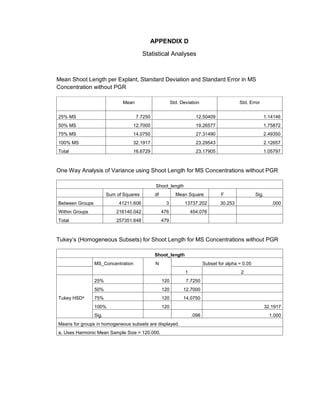
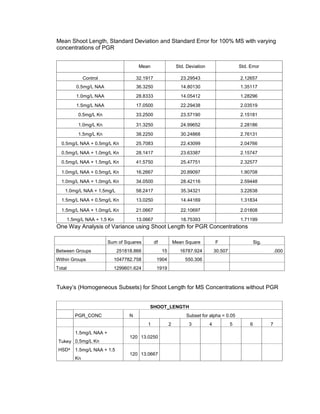
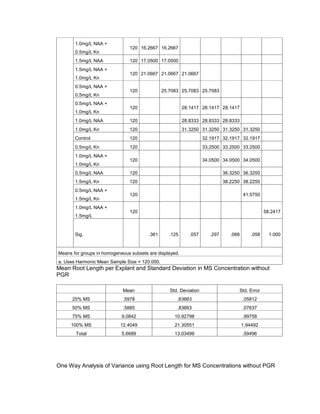
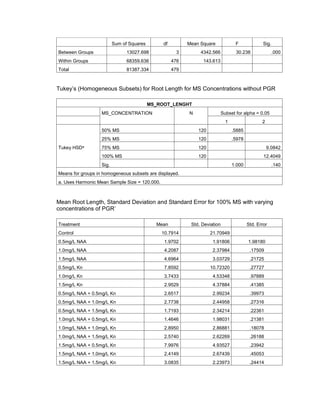
This document is a thesis presented to the faculty of the Department of Biology at the Polytechnic University of the Philippines by Tiu, Lualhati S.D. and Javier, Amalia Carla Severina S. in partial fulfillment of the requirements for the degree of Bachelor of Science in Biology. The thesis examines the in vitro cultivation of the Philippine native variety of Allium sativum Linn. (Ilocos White). Various concentrations of Murashige and Skoog media were tested, with and without the addition of plant growth regulators kinetin and NAA. The explants underwent direct organogenesis without a callus phase. Shoot and root formation and growth were evaluated under different treatment conditions.