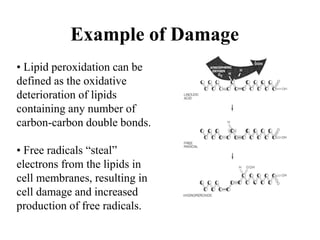
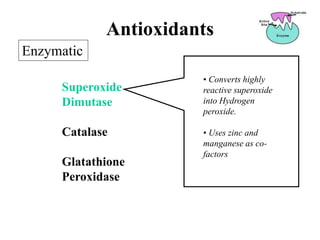
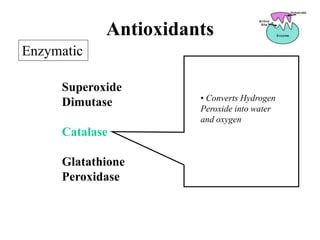
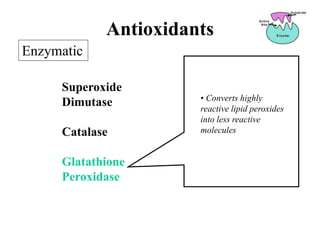
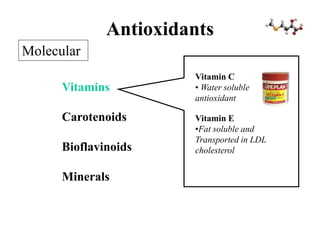
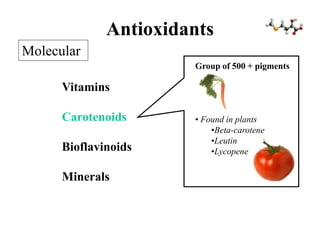
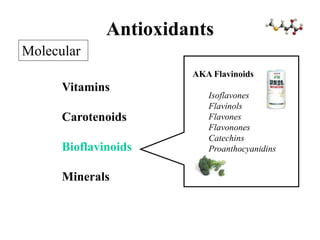
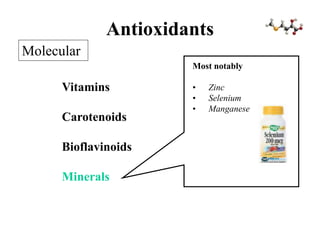
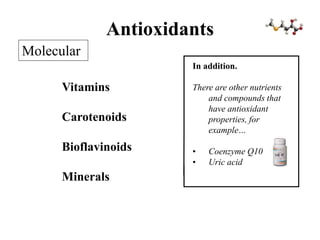
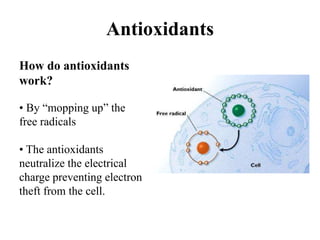
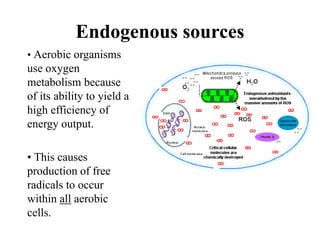
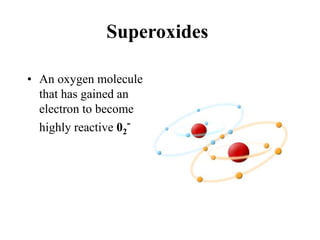
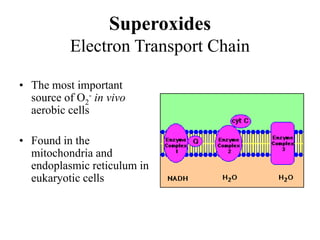
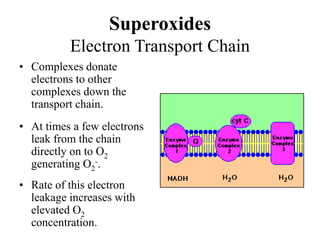
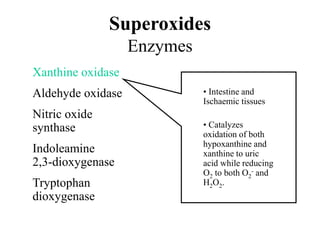
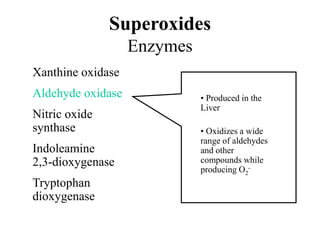
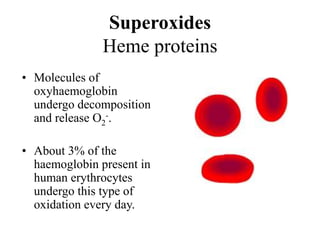
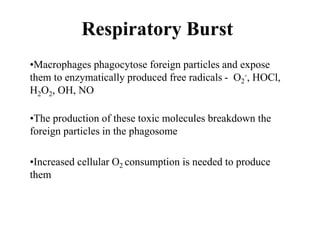
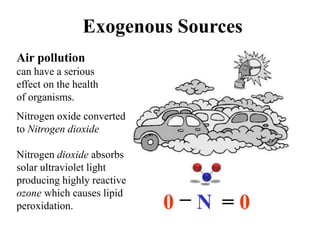
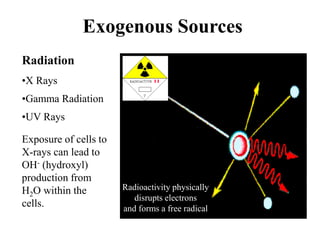
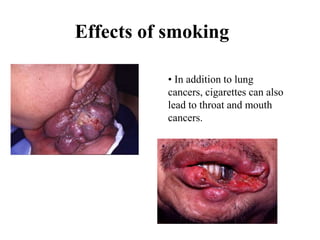
Free radicals are molecules with unpaired electrons that can cause damage by reacting with other molecules to steal electrons. They are produced endogenously through metabolism but also come from exogenous sources like pollution, radiation, and cigarettes. Free radical damage at the cellular and molecular level can lead to health issues including cancer and aging. The impact of free radicals can be reduced by decreasing exposure to sources, exercising to boost antioxidant defenses, eating antioxidant-rich foods, and taking antioxidant supplements.


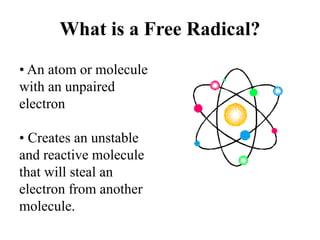
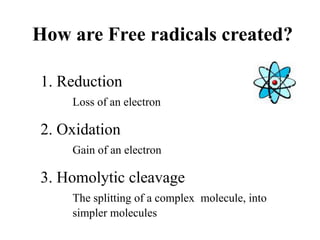




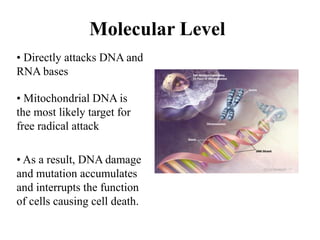
![Cellular Level
• Unsaturated lipid molecules
of cell membranes are
particularly susceptible to
damage.
• Free radicals breakdown of
lipids resulting in the
hardening of cell wall.
• This can lead to a decrease
intracellular [Ca2+] which
results in reduced neural
activity.](https://image.slidesharecdn.com/freeradicals-240328035151-b6ceb0f8/85/Free-Radicals-CHEMISTRY-presentation-ppt-31-320.jpg)