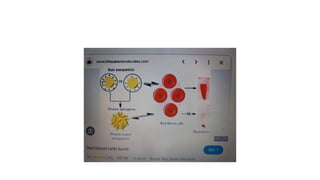
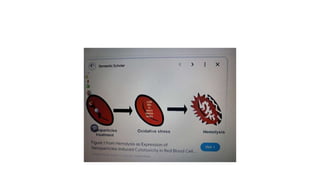
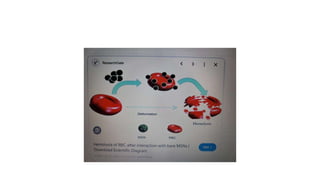
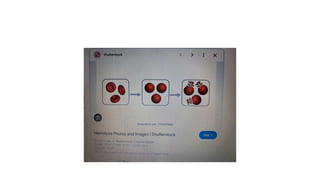
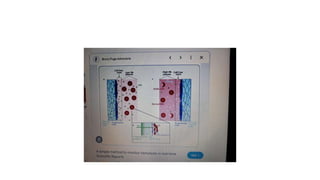
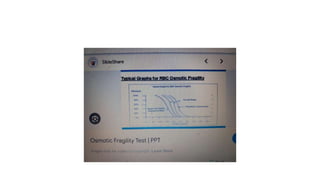
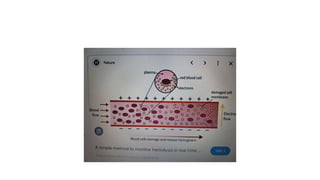
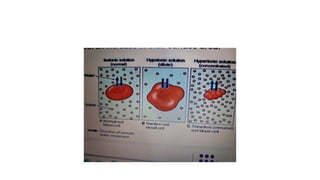
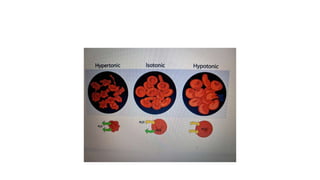
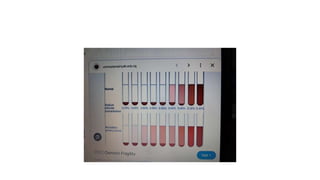
The fragility test measures the resistance of erythrocytes in hypotonic saline solutions, using various concentrations of sodium chloride. Results are determined by visual and centrifugation methods, indicating the degree of hemolysis based on the clarity and color of the fluid. Factors leading to hemolysis include hemolytic jaundice, certain chemicals, and bacterial toxins.












![• REFERENCES:-
• Pagana KD, Pagana TJ, Pagana TN. Mosby's Diagnostic & Laboratory Test Reference. 14th ed. St. Louis,
MO: Elsevier; 2019. 371.
• Parpart AK, Lorenz PB, Parpart ER, Gregg JR, Chase AM. The osmotic resistance (fragilità) of human red
cells. J Clin Invest. 1947. 26:636-40.
• Yamamoto A, Saito N, Yamauchi Y, Takeda M, Ueki S, Itoga M, et al. Flow Cytometric Analysis of Red
Blood Cell Osmotic Fragility. J Lab Autom. 2014 Apr 21. [QxMD MEDLINE Link].
• Walski T, Chludzinska L, Komorowska M, Witkiewicz W. Individual osmotic fragility distribution: a new
parameter for determination of the osmotic properties of human red blood cells. Biomed Res Int. 2014.
2014:162102. [QxMD MEDLINE Link]. [Full Text].
• Gallagher PG. The Red Blood Cell Membrane and Its Disorders: Hereditary Spherocytosis, Elliptocytosis,
and Related Diseases. Prchal JT, Kaushansky K, Lichtman MA, Kipps TJ, Seligsohn U, eds. Williams
Hematology 2010. 8th ed. New York: McGraw-Hill; September 5, 2012. Chapter 45. [Full Text].
• Kattamis C, Efremov G, Pootrakul S. Effectiveness of one tube osmotic fragility screening in detecting beta-
thalassaemia trait. J Med Genet. 1981 Aug. 18(4):266-70. [QxMD MEDLINE Link]](https://image.slidesharecdn.com/fragilitytestcausesdiagnosisalleviation-240611052828-41d7d633/85/Fragility-test_causes_diagnosis_alleviation-pptx-26-320.jpg)