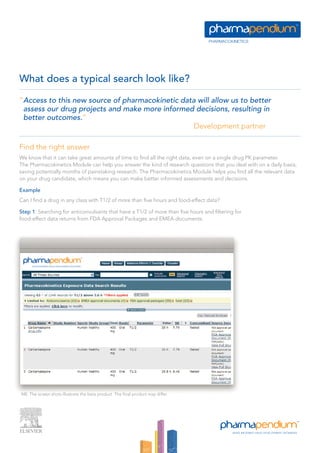
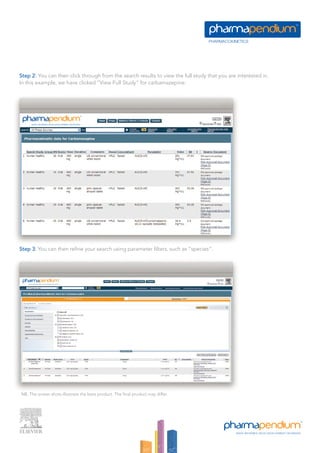
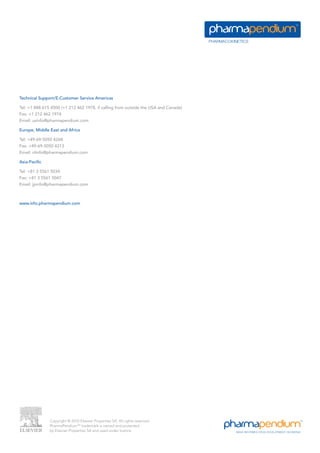
The pharmacokinetics module provides unprecedented access to a vast database of pharmacokinetic data from various regulatory sources, enabling professionals to make better-informed drug development decisions. Users can search and filter data based on numerous parameters, including drug exposure, dose, and demographic differences, thus streamlining their research efforts. This module supports improved project risk assessments and competitive positioning by delivering insights on therapeutic candidates and their pharmacokinetic profiles.