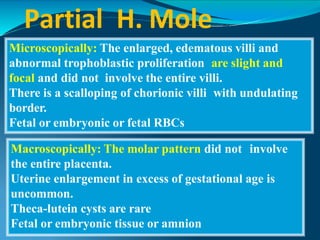
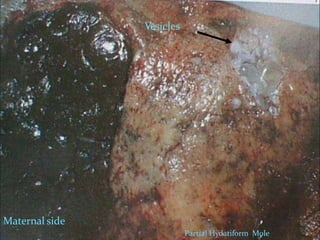
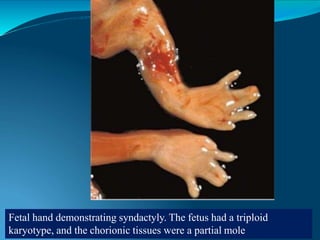
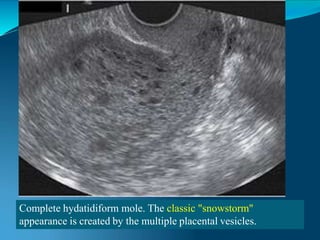
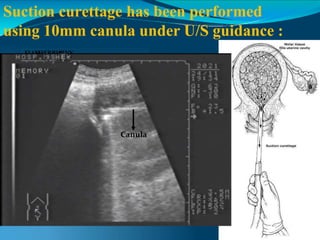
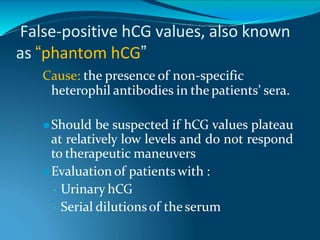
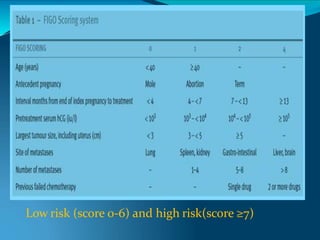
This document discusses molar pregnancy, which occurs when abnormal placental tissue develops instead of an embryo. It defines molar pregnancy and describes the classification of gestational trophoblastic disease. It also covers the pathogenesis, risk factors, clinical features, diagnosis, and management of complete and partial molar pregnancies. The key aspects are evacuation of the molar tissue followed by monitoring of hCG levels to detect persistent trophoblastic disease.