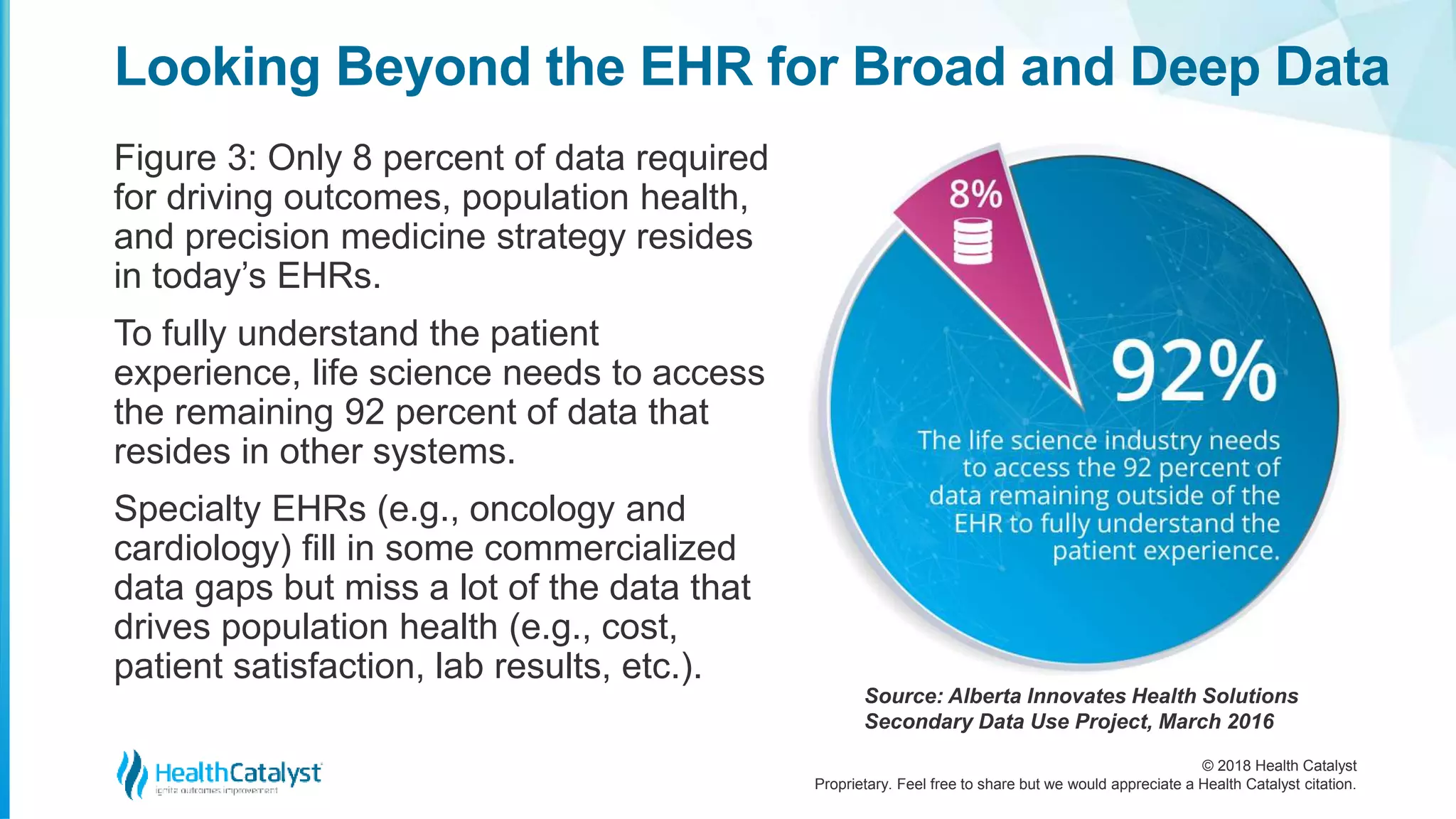
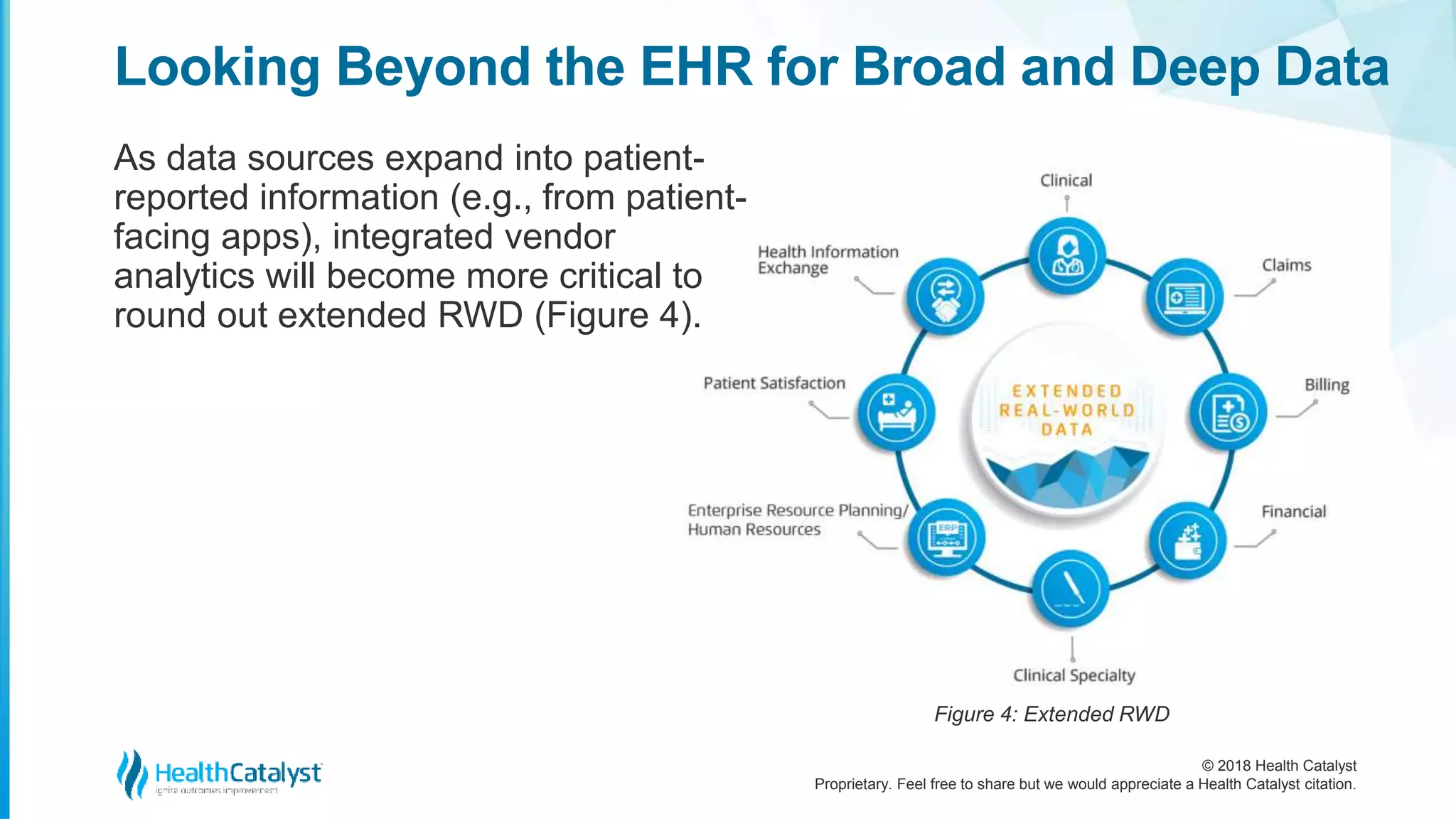
The document discusses the significance of extended real-world data (RWD) and real-world evidence (RWE) in the life sciences industry, particularly for improving drug development processes. It emphasizes the need for life science companies to integrate diverse patient-centric data to enhance clinical trials, patient outcomes, and overall efficiency in the healthcare system. Additionally, it highlights the crucial role of partnerships with healthcare transformation organizations to effectively leverage data for actionable insights and real-world applications.

















![© 2018 Health Catalyst
Proprietary. Feel free to share but we would appreciate a Health Catalyst citation.
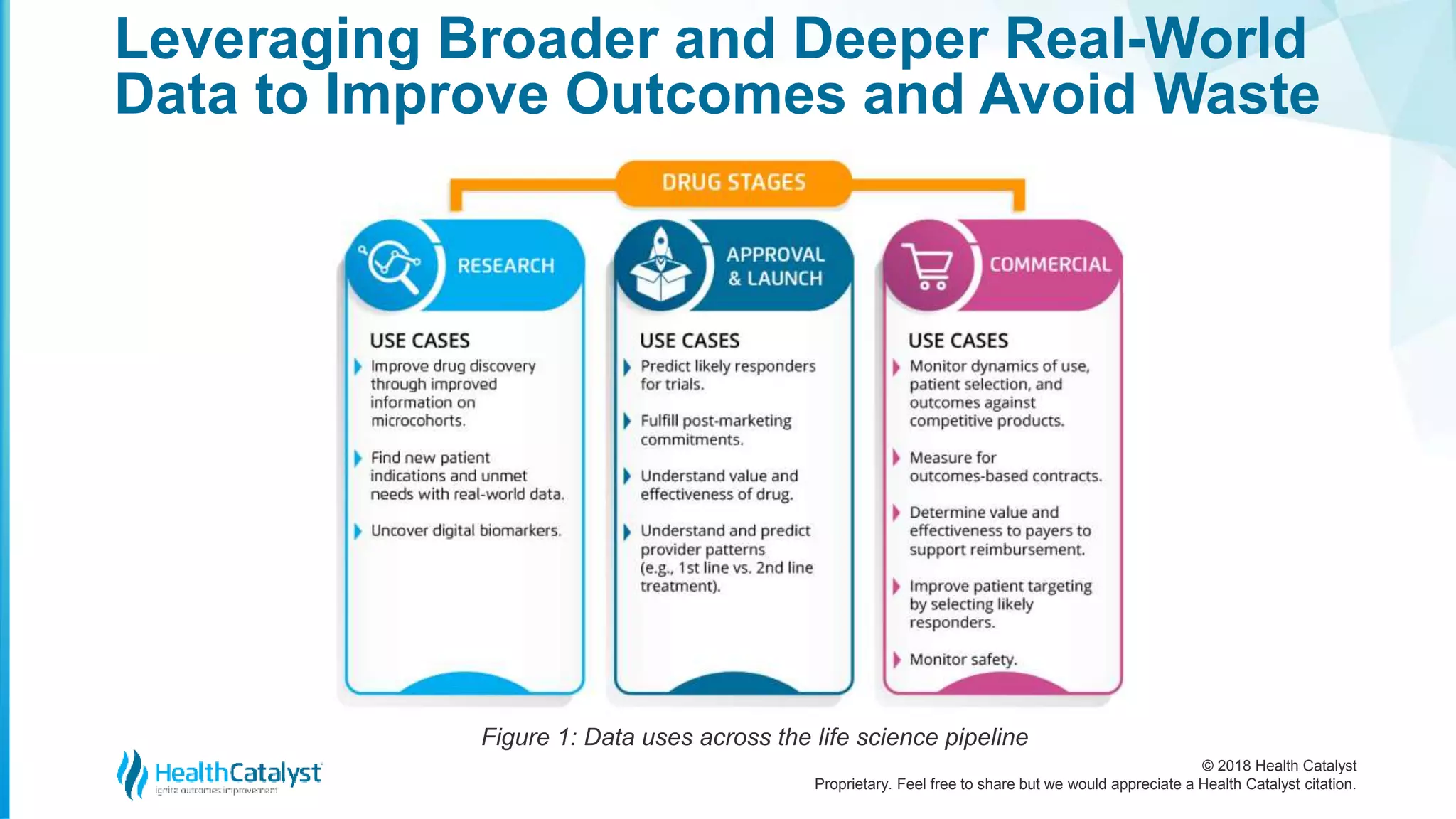
Leveraging Broader and Deeper Real-World
Data to Improve Outcomes and Avoid Waste
Offerings have started to grow
around certain therapeutic
areas, and healthcare analytics
vendors are now expanding
offerings to meet the demand
for integrated data from many
sources (e.g., labs, consumer
behavior, and more [Figure 2])
that capture the breadth of
patient health.
Figure 2: Life science companies are interested in a variety of data](https://image.slidesharecdn.com/extendedreal-worlddata-thelifescienceindustrysnumberoneasset-190401165011/75/Extended-Real-World-Data-The-Life-Science-Industry-s-Number-One-Asset-20-2048.jpg)