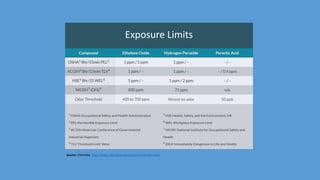
These documents discuss hospitals switching from high-level disinfection to ethylene oxide (EtO) gas sterilization of duodenoscopes in response to carbapenem-resistant Enterobacteriaceae (CRE) outbreaks. Investigations found the outbreaks were linked to contaminated duodenoscopes despite following reprocessing protocols. Hospitals that replaced high-level disinfection with EtO sterilization or implemented post-reprocessing testing did not have additional multidrug-resistant infections. The FDA and hospitals recommend EtO gas sterilization for reliably eliminating contamination from duodenoscopes.