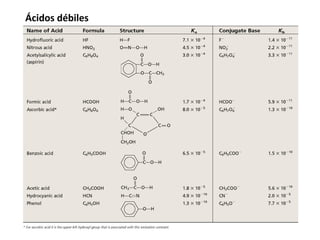
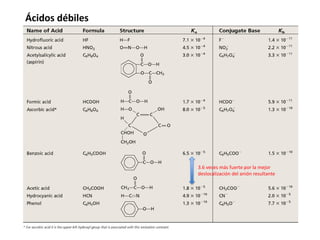
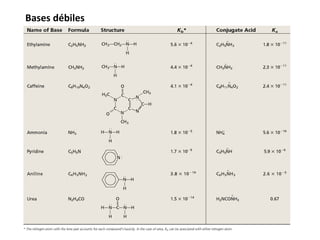
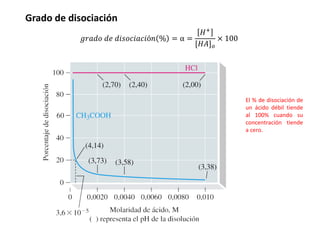
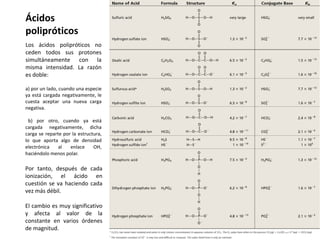
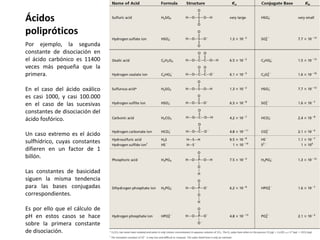
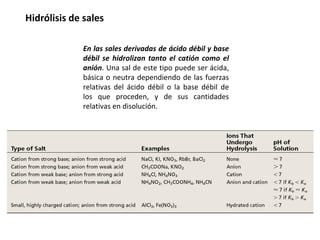
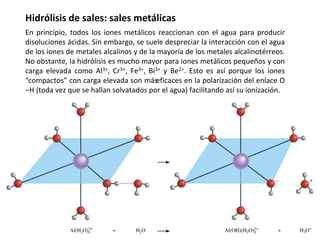
This document discusses acid-base equilibria and reactions in aqueous solution. It begins by explaining that chemical processes progress from reactants to products at decreasing rates until equilibrium is reached when the rates of product formation and decomposition back to reactants are equal. It then provides introductions to theories of acids and bases according to Arrhenius and Brønsted-Lowry, including autoionization of water and the definition of pH. The document discusses factors that determine acid strength such as molecular structure, ionization constants, and the degree of dissociation of weak acids. It also covers polyprotic acids, hydrolysis of salts, and acid-base indicators.




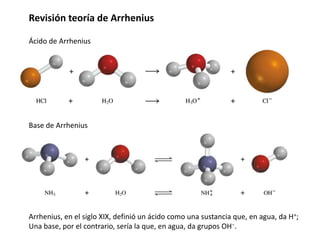
![Autoionización y producto iónico del agua
O
H
H + O
H
H O
H
H H O
H
−
+
+
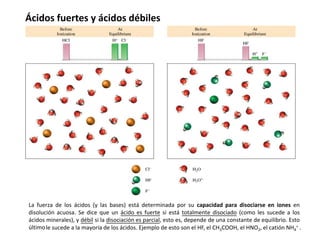
H2O + H2O H3O+ + OH−
ácido base
conjugada
base
ácido
conjugado
]
[
El agua puede reaccionar consigo misma en un proceso ácido base para dar
protones e hidróxidos simultáneamente.
Que se puede interpretar según la teoría de Brønsted como:
KW = [H+][OH−] = 1.0 x 10-14
Producto iónico del agua
En condiciones estándar](https://image.slidesharecdn.com/sesion07-equilibrioacido-base-210421135422/85/Equilibrio-acido-base-8-320.jpg)
![Definición de pH y pOH
pH = −log [H+]
pOH = −log [OH−]
[H+][OH−] = KW = 1.0 x 10-14
–log [H+] – log [OH−] = 14.00
pH + pOH = 14.00](https://image.slidesharecdn.com/sesion07-equilibrioacido-base-210421135422/85/Equilibrio-acido-base-9-320.jpg)







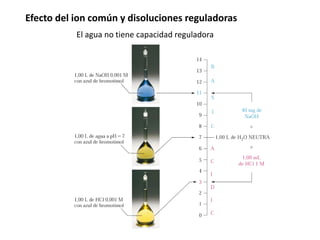
![Efecto del ion común y disoluciones reguladoras
Una disolución reguladora ofrece su máxima capacidad reguladora del pH cuando las
Concentraciones del ácido y la base conjugadas son iguales. En cualquier caso, puede
ejercer esta función cuando la relación es 0.1 < [A−]/[HA] < 10](https://image.slidesharecdn.com/sesion07-equilibrioacido-base-210421135422/85/Equilibrio-acido-base-30-320.jpg)


![Efecto del ion común y disoluciones reguladoras
Problema. Sabiendo que el valor de Ka del ácido acético es 1.75 × 10−5 a 20 oC.
a) Calcular el pH de 1 L de una disolución 0.1 M en ácido acético y 0.1 M en acetato de
sodio.
𝑝𝐻 = 4.76
b) Calcular nuevamente el pH de la disolución cuando: i) se añaden 10 mL de ácido clorhídrico
0.1 M, y ii) cuando se añaden 10 mL de hidróxido de sodio 0.1 M.
i) 10 mL 0.1 M son 0.001 moles de HCl. Al añadir el ácido, este reacciona
con la base presente, el acetato, protonándolo y generando más acético.
De esta forma, las nuevas concentraciones serán:
[acetato] = 0.099 moles/1.01 L = 0.098 M
[acético] = 0.101 moles/1.01 L = 0.1 M
𝑝𝐻 = 4.76 + 𝑙𝑜𝑔
0.098
0.1
= 4.75
¡Esa misma cantidad de ácido en 1 L de agua pura, daría un pH = 3.00!](https://image.slidesharecdn.com/sesion07-equilibrioacido-base-210421135422/85/Equilibrio-acido-base-33-320.jpg)
![Efecto del ion común y disoluciones reguladoras
Problema. Sabiendo que el valor de Ka del ácido acético es 1.75 × 10−5 a 20 oC.
a) Calcular el pH de 1 L de una disolución 0.1 M en ácido acético y 0.1 M en acetato de
sodio.
𝑝𝐻 = 4.76
b) Calcular nuevamente el pH de la disolución cuando: i) se añaden 10 mL de ácido clorhídrico
0.1 M, y ii) cuando se añaden 10 mL de hidróxido de sodio 0.1 M.
ii) 10 mL 0.1 M son 0.001 moles de NaOH. Al añadir la base, esta reacciona
con el ácido presente, el acético, desprotonándolo y generando más acetato.
De esta forma, las nuevas concentraciones serán:
[acetato] = 0.101 moles/1.01 L = 0.1 M
[acético] = 0.099 moles/1.01 L = 0.098 M
𝑝𝐻 = 4.76 + 𝑙𝑜𝑔
0.1
0.098
= 4.77
¡Esa misma cantidad de base en 1 L de agua pura, daría un pH = 11.00!](https://image.slidesharecdn.com/sesion07-equilibrioacido-base-210421135422/85/Equilibrio-acido-base-34-320.jpg)




![ 𝑆𝑖:
𝐾𝑤 = 𝑂𝐻−
. [𝐻3𝑂+
]
[𝐻3𝑂+] =
𝐾𝑤
𝑂𝐻−
𝑂𝐻− =
𝐾𝑤
𝐻3𝑂+
𝐻3𝑂+
= 𝐶𝑎 +
𝐾𝑤
𝐻3𝑂+
𝐶𝑎 ≫
𝐾𝑤
𝐻3𝑂+
𝐶𝑎 ≪
𝐾𝑤
𝐻3𝑂+
𝑆𝑖:
𝐻3𝑂+
= 𝐶𝑎
𝐻3𝑂+
= 𝐶𝑎 +
𝐾𝑤
𝐻3𝑂+](https://image.slidesharecdn.com/sesion07-equilibrioacido-base-210421135422/85/Equilibrio-acido-base-50-320.jpg)
![EJEMPLO 01: Hallar el pH de una solución de HClO4 10-8 M.
Solución:
[𝐻3𝑂+
] = 𝐶𝑙𝑂4
−
+ [𝑂𝐻−
]
𝐻𝐶𝑙𝑂4 + 𝐻2𝑂 ⇆ 𝐻3𝑂(𝑎𝑐)
+
+ 𝐶𝑙𝑂4(𝑎𝑐)
−
𝐶𝑎 = 𝐻3𝑂+
= [𝐵𝑎𝑠𝑒 𝑐𝑜𝑛𝑗𝑢𝑔𝑎𝑑𝑎]
𝐵𝑀
𝐵𝐶
𝐾𝑤 = 𝑂𝐻−
. [𝐻3𝑂+
]
[𝐻3𝑂+] =
𝐾𝑤
𝑂𝐻− 𝑂𝐻− =
𝐾𝑤
𝐻3𝑂+
[𝐻3𝑂+
] = 𝐶𝑎 +
𝐾𝑤
𝐻3𝑂+
[𝐻3𝑂+] = 10−8 +
1 × 10−14
𝐻3𝑂+
Resolviendo la ecuación de segundo grado:
[𝐻3𝑂+] = 1,05 × 10−7
𝑝𝐻 = − lo𝑔[𝐻3𝑂+
]
𝑝𝐻 = − lo𝑔 1,05 × 10−7
𝑝𝐻 = 6,98](https://image.slidesharecdn.com/sesion07-equilibrioacido-base-210421135422/85/Equilibrio-acido-base-51-320.jpg)
![EJEMPLO 02: Se tiene una solución de HNO3 0,01 M. Hallar el pH.
Solución:
𝑁𝑂3
−
= 𝐻3𝑂+
𝐻𝑁𝑂3(𝑎𝑐) + 𝐻2𝑂(𝑙) ⇆ 𝐻3𝑂(𝑎𝑐)
+
+ 𝑁𝑂3(𝑎𝑐)
−
𝐷𝑖𝑠𝑜𝑐𝑖𝑎𝑐𝑖ó𝑛 𝑐𝑜𝑚𝑝𝑙𝑒𝑡𝑎
[𝐻3𝑂+] = 𝐶𝑎 +
𝐾𝑤
𝐻3𝑂+
[𝐻3𝑂+] = 0,01 +
1 × 10−14
𝐻3𝑂+
Resolviendo la ecuación de segundo grado:
[𝐻3𝑂+] = 0,01
𝑝𝐻 = − lo𝑔[𝐻3𝑂+]
𝑝𝐻 = − lo𝑔 0,01
𝑝𝐻 = 2
0,01 𝑀](https://image.slidesharecdn.com/sesion07-equilibrioacido-base-210421135422/85/Equilibrio-acido-base-52-320.jpg)
![EJEMPLO 03: El pH de una solución acuosa es 12,6. ¿Cuál será la [OH-] y el pOH a la
temperatura de 25°C
Solución:
𝑝𝐻 = − lo𝑔[𝐻3𝑂+] = 12,6
𝐻3𝑂+
= 10−𝑝𝐻
= 10−12,6
𝑀
𝐾𝑤 = 𝐻3𝑂+ . 𝑂𝐻− = 1 × 10−14
𝐻3𝑂+ = 2,5 × 10−13 𝑀
𝑂𝐻−
=
𝐾𝑤
𝐻3𝑂+
𝑂𝐻− =
1 × 10−14 𝑀2
2,5 × 10−13 𝑀
𝑂𝐻−
= 0,04 𝑀
𝑝𝑂𝐻 = − lo𝑔 𝑂𝐻−
𝑝𝑂𝐻 = − lo𝑔 0,04 𝑀
𝑝𝑂𝐻 = 1,4
Comprobando:
𝑝𝐻 + 𝑝𝑂𝐻 = 14
12,6 + 1,4 = 14
14 = 14](https://image.slidesharecdn.com/sesion07-equilibrioacido-base-210421135422/85/Equilibrio-acido-base-53-320.jpg)
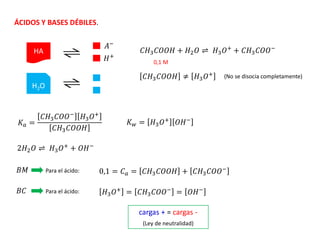
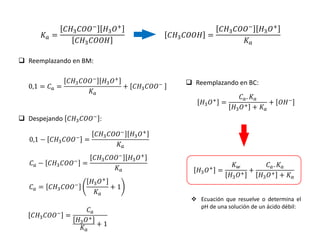
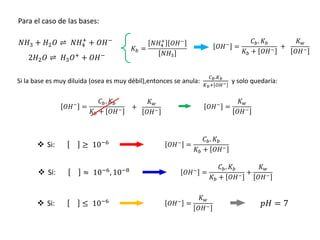
![ Cuando es un ácido muy débil, de tal forma que nos proporciona una cantidad de iones H+
despreciable o cuando se tiene una solución de ácido muy diluida, se tiene:
𝐾𝑤
𝐻3𝑂+
≫
𝐶𝑎. 𝐾𝑎
𝐻3𝑂+ + 𝐾𝑎
10-5, 10-8, 10-12
Entre menor sea la constante de
disociación, el ácido será más débil
𝐻3𝑂+
=
𝐾𝑤
𝐻3𝑂+
𝐻3𝑂+
= 𝐾𝑤
𝐻3𝑂+
= 10−7
𝑝𝐻 = 7
-7
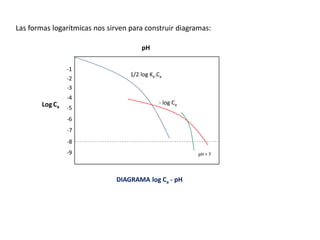
DIAGRAMA DE FLOOD
Log
.
[
]
Ácido fuerte
-1
-2
-3
-4
-5
-6
-8
-9
pH = 7
Sólo
depende
del agua
pendiente
negativa
Entre mas se disocia el ácido; Ka
aumenta: [H+] = 10-7 ; pH = 7
En ácidos muy débiles la 𝐻3𝑂+
que
aporta es despreciable frente al solvente,
entonces se tiene que el pH es 7; entonces
se considera como si fuera agua pura
𝐻3𝑂+ = 𝐾𝑤](https://image.slidesharecdn.com/sesion07-equilibrioacido-base-210421135422/85/Equilibrio-acido-base-56-320.jpg)
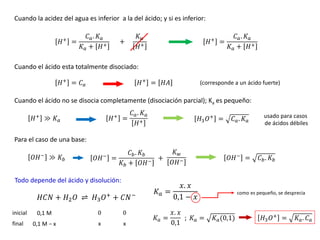
![EJEMPLO 01: Calcular el pH de una solución que contiene 2,5 g de NaOH en 300 mL de
solución.
Solución:
𝑃 ഥ
𝑀𝑁𝑎𝑂𝐻 = 40 𝑔/𝑚𝑜𝑙
𝑝𝐻 =¿ ?
𝑉 = 300 𝑚𝐿
𝑀 =
2,5 𝑔 𝑁𝑎𝑂𝐻
40 𝑔/𝑚𝑜𝑙 𝑁𝑎𝑂𝐻
0,3 𝐿
𝑀 = 0,208 𝑚𝑜𝑙/𝐿 𝑂𝐻− = 0,208 𝑀
𝑝𝑂𝐻 = −𝑙𝑜𝑔 𝑂𝐻−
𝑝𝑂𝐻 = − log(0,208)
𝑝𝑂𝐻 = 0,68
𝑝𝐻 + 𝑝𝑂𝐻 = 14
𝑝𝐻 + 0,68 = 14
𝑝𝐻 = 13,32
Calculando la [OH-]:
Calculando el PH:](https://image.slidesharecdn.com/sesion07-equilibrioacido-base-210421135422/85/Equilibrio-acido-base-58-320.jpg)
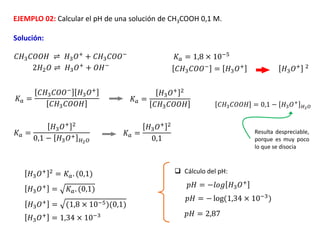
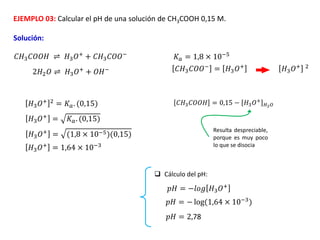
![EJEMPLO 04: Hallar el pH de las siguientes soluciones:
a) Una solución de NaOH 0,2 M
b) Una solución de NH4OH 0,2 M
𝑁𝑎𝑂𝐻 → 𝑁𝑎+
+ 𝑂𝐻−
NaOH es una base fuerte; eso quiere decir que se
disocia completamente; por lo tanto [OH-] = Cb
𝑂𝐻− = 𝐶𝑏 = 0,2 𝑀
𝑝𝑂𝐻 = −𝑙𝑜𝑔 𝑂𝐻−
𝑝𝑂𝐻 = −𝑙𝑜𝑔 (0,2)
𝑝𝑂𝐻 = 0,69
𝑝𝐻 = 13,31
𝑁𝐻4𝑂𝐻 ⇌ 𝑁𝐻4
+
+ 𝑂𝐻−
NH4OH es una base débil; eso quiere decir que no
se disocia completamente; por lo tanto [OH-] ≠ Cb
𝑁𝐻4
+
= 𝑂𝐻−
𝐾𝑏 = 1,75 × 10−5
𝑂𝐻− = 𝐾𝑏. 𝐶𝑏
𝑂𝐻−
= (1,75 × 10−5)(0,2)
𝑂𝐻−
= 1,87 × 10−3
𝑝𝑂𝐻 = −𝑙𝑜𝑔 𝑂𝐻−
𝑝𝑂𝐻 = −𝑙𝑜𝑔(1,87 × 10−3) 𝑝𝑂𝐻 = 2,72 𝑝𝐻 = 11,28](https://image.slidesharecdn.com/sesion07-equilibrioacido-base-210421135422/85/Equilibrio-acido-base-62-320.jpg)