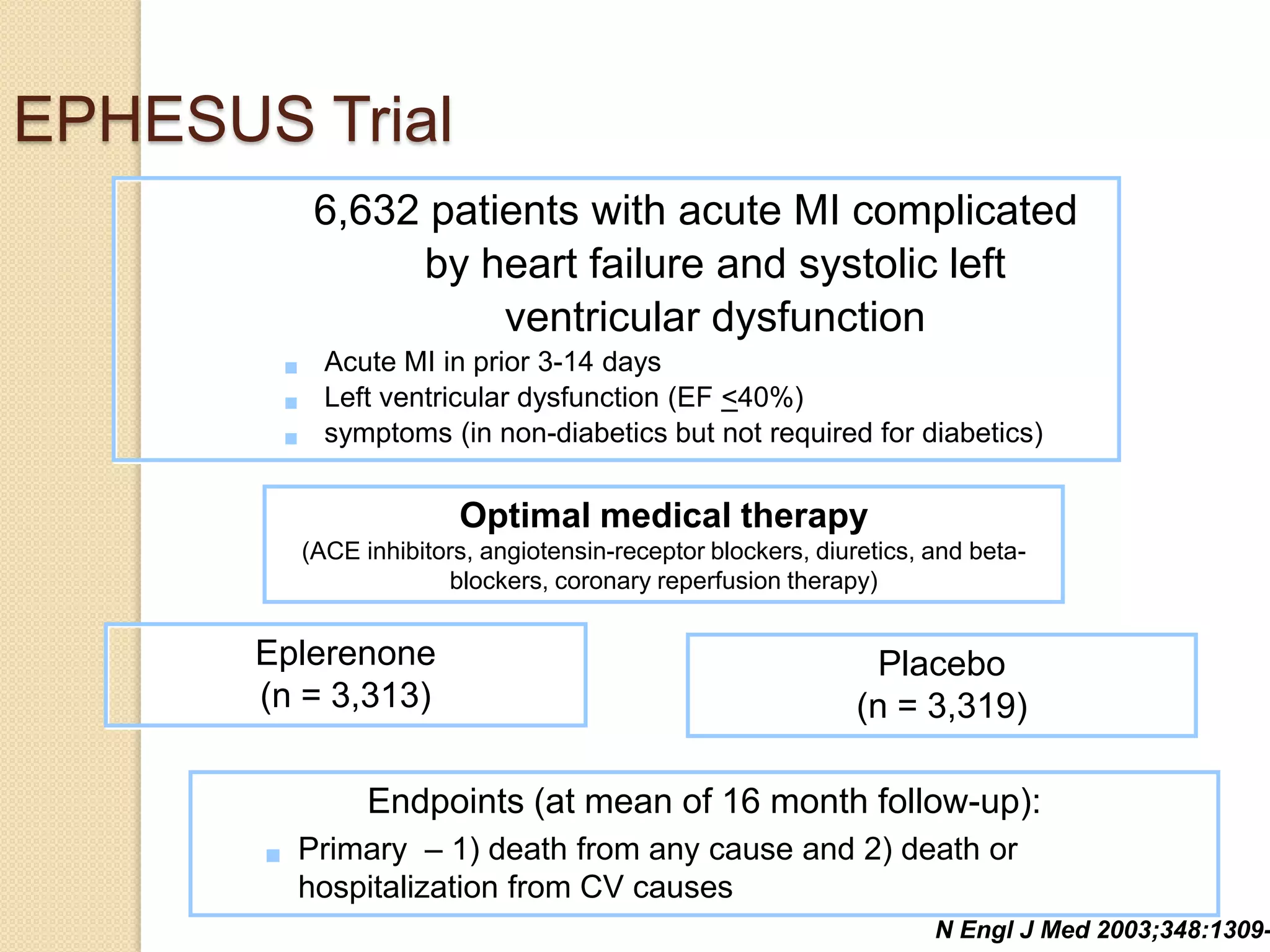
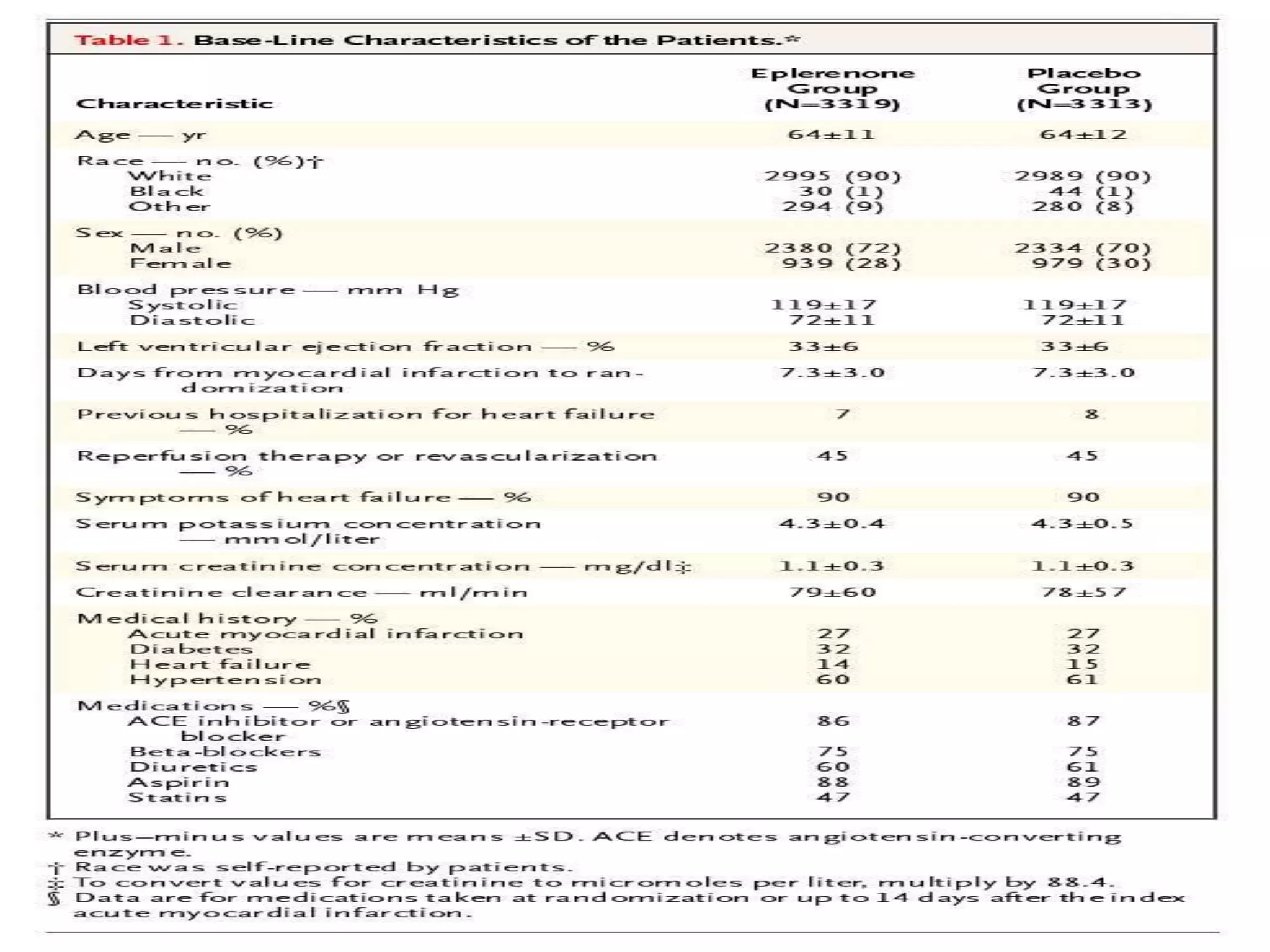
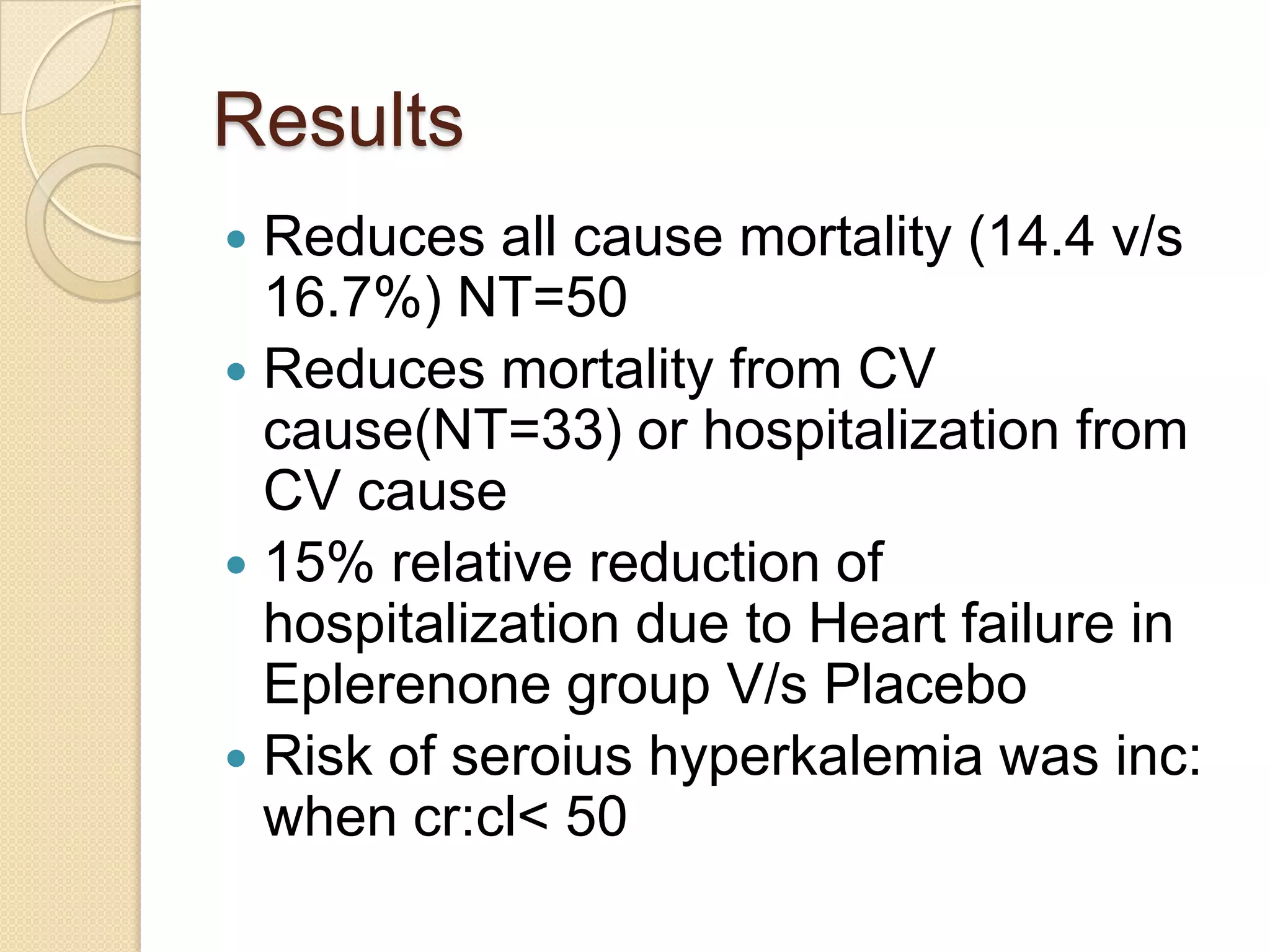
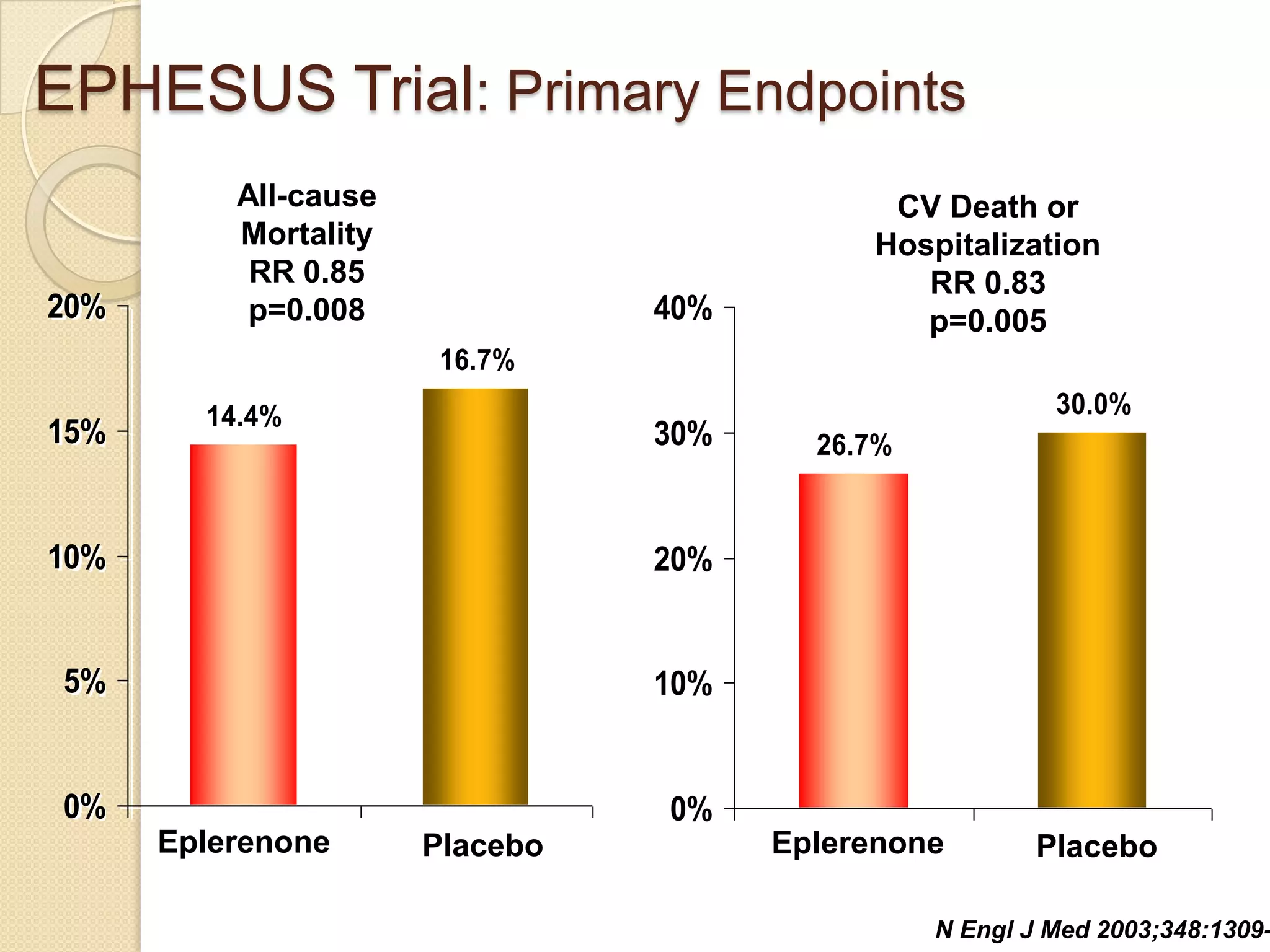
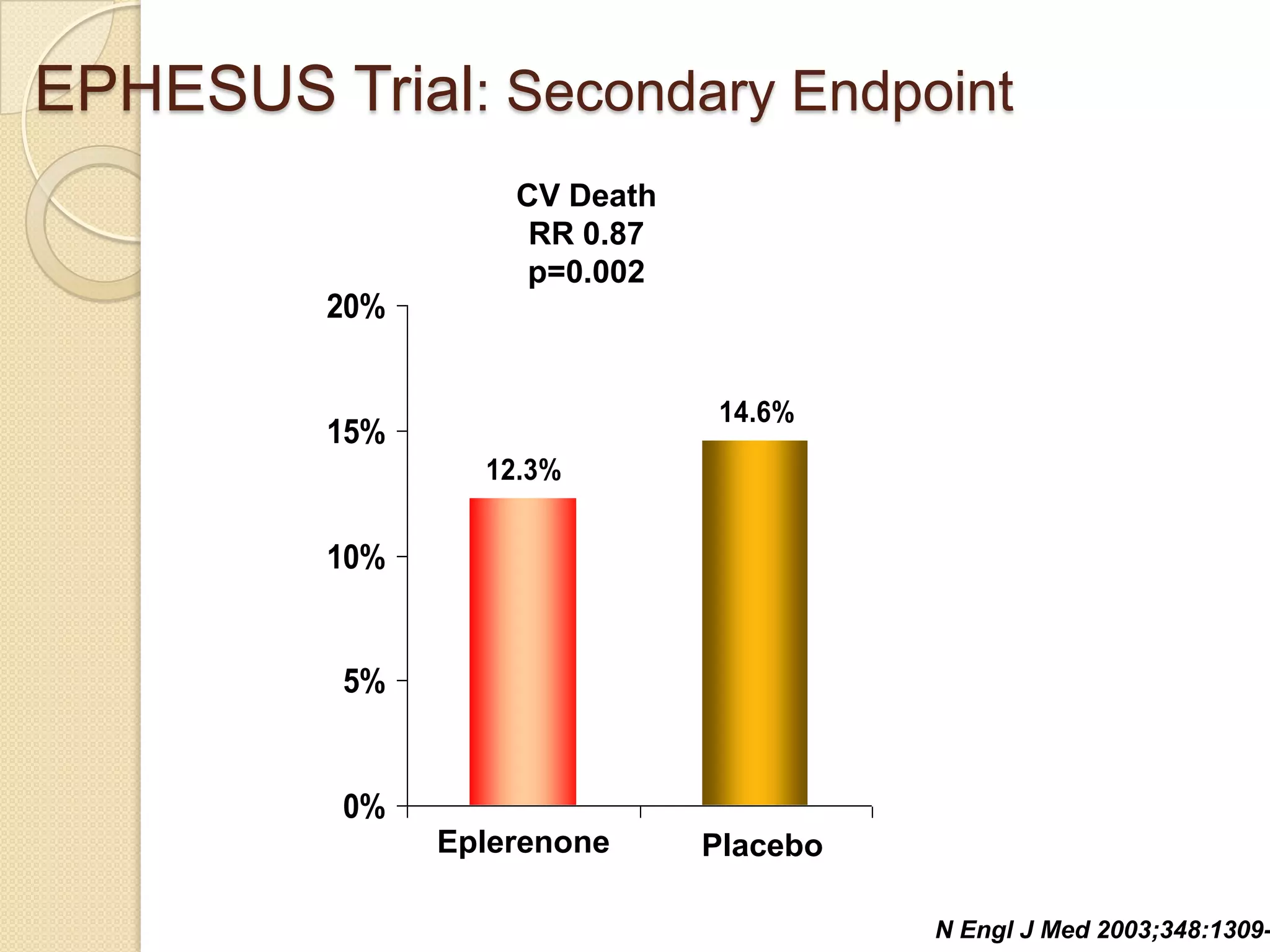
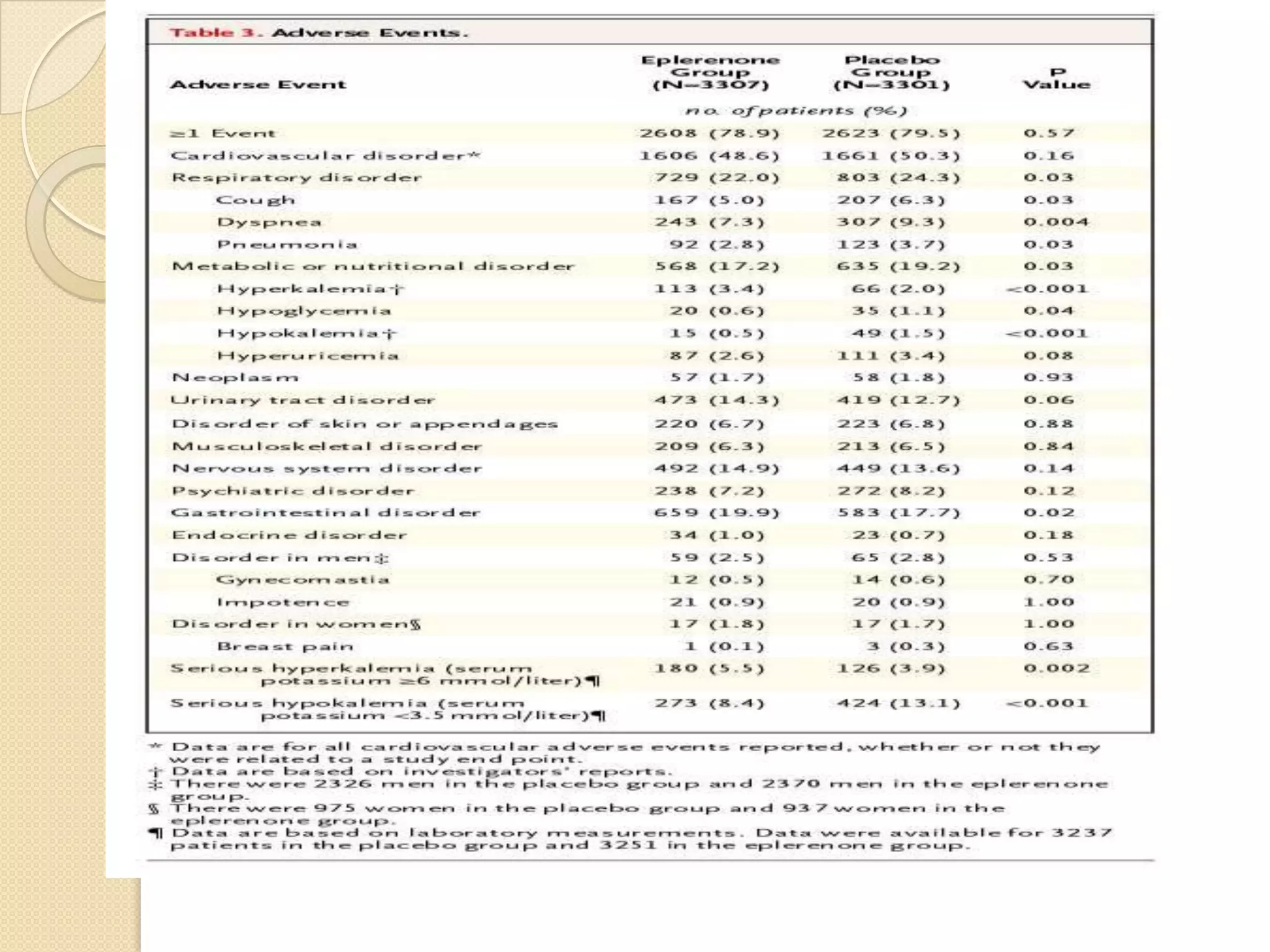
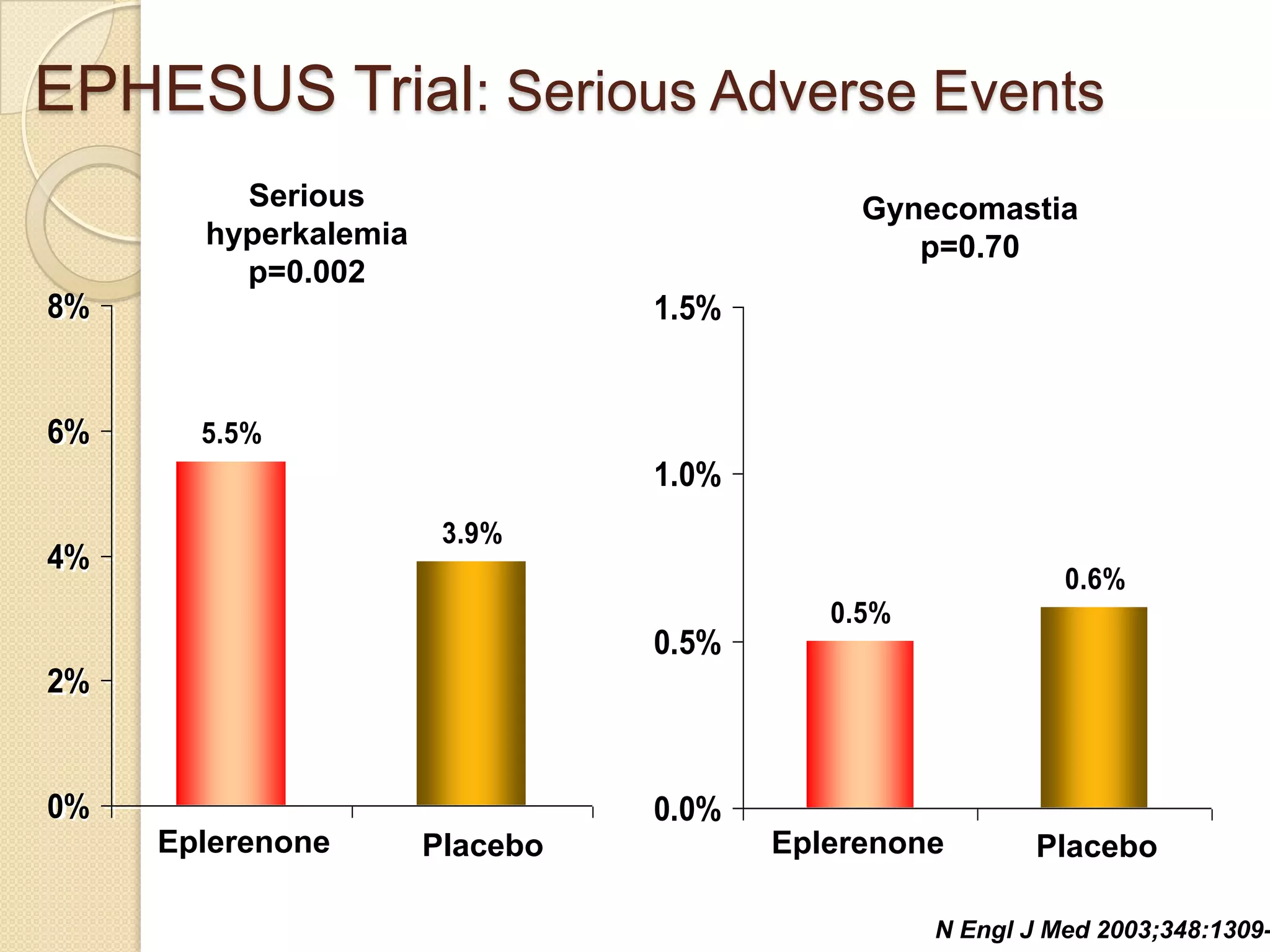
This document summarizes a clinical trial that evaluated the effect of the selective aldosterone blocker eplerenone in patients with left ventricular dysfunction after myocardial infarction. The trial involved over 6,600 patients who received either eplerenone or a placebo in addition to optimal medical therapy. The results showed that eplerenone reduced all-cause mortality by 14.4% compared to 16.7% for placebo and reduced cardiovascular mortality and hospitalization. However, there was an increased risk of serious hyperkalemia with eplerenone. The study concluded that eplerenone improves outcomes for patients with left ventricular dysfunction after a myocardial infarction when added to maximal medical therapy.