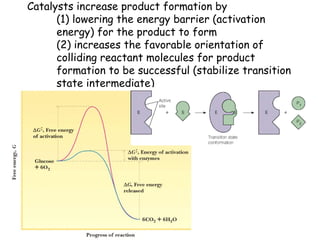
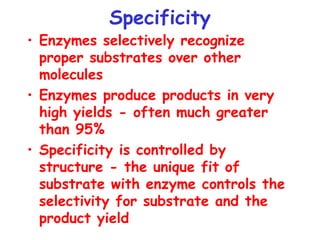
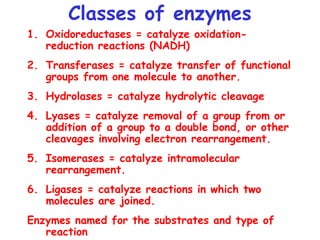
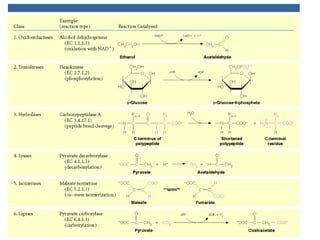
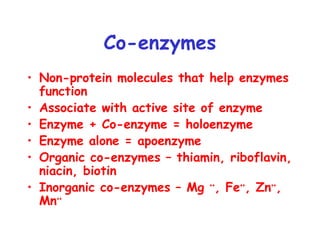
Chapter 5 discusses enzymes as catalysts that accelerate chemical reactions without altering equilibrium, significantly increasing product formation by lowering activation energy and enhancing reactant orientation. Enzymes are highly specific, producing products in yields exceeding 95% and are categorized into classes like oxidoreductases and transferases, based on their functions. The chapter also covers co-enzymes that assist enzymes and the principles of kinetics in understanding reaction rates and mechanisms.