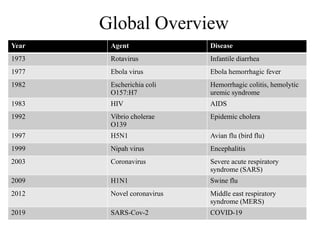
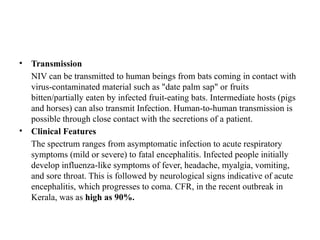
Emerging and re-emerging infectious diseases are major public health concerns in the 21st century. These diseases challenge health systems, disrupt economies, and cause significant morbidity and mortality globally. With globalization, climate change, urbanization, and antimicrobial resistance on the rise, the threat posed by these diseases is growing. Infectious diseases are caused by pathogenic microorganisms such as bacteria, viruses, parasites, or fungi. They can spread, directly or indirectly, from one person to another. Over time, new diseases can emerge, or previously controlled ones can reappear, often with greater virulence or resistance.