This document provides information on the principles and techniques of electrophoresis. It begins with an introduction to electrophoresis as the migration of charged particles under an electric field. It then discusses various factors that affect electrophoresis like net charge, size, shape, buffer properties, and temperature.
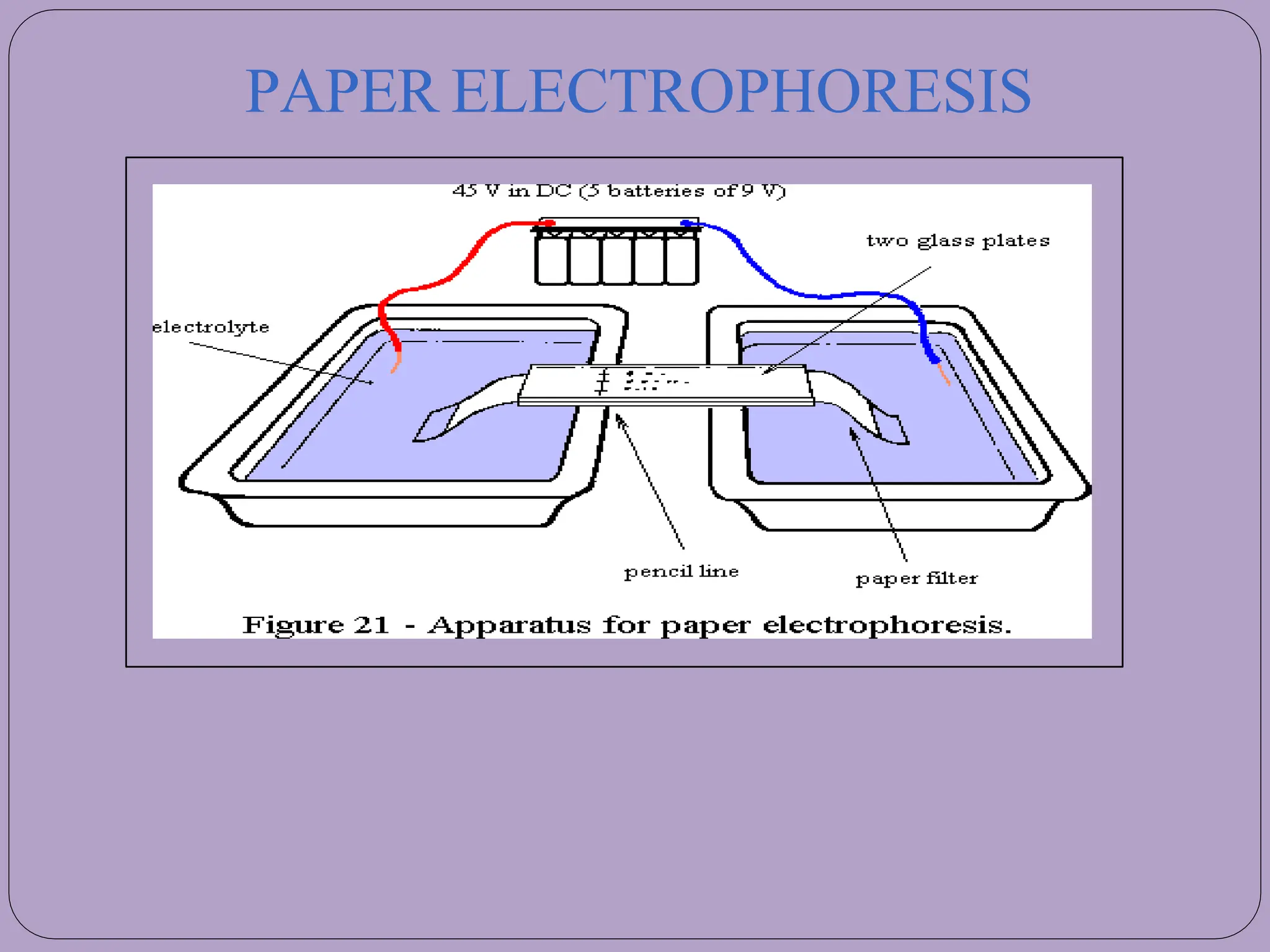
The document goes on to describe different types of electrophoresis techniques in detail, including paper electrophoresis, gel electrophoresis using agarose or polyacrylamide gels, SDS-PAGE, isoelectric focusing, two-dimensional polyacrylamide gel electrophoresis, isotachophoresis, pulsed-field gel electrophoresis, and immunoelectrophoresis. It explains the basic principles, applications, advantages and disadvantages of each technique.





















































![Movementofparticle
[Cl]> [protein-SDS]> [Glycinate]
54](https://image.slidesharecdn.com/electrophoresis-ppt-240318115911-4e3a7e8f/75/ELECTROPHORESIS-and-its-types-ppt-pptx-55-2048.jpg)





















![-Precipitation reactions arebasedon the interaction of antibodies andantigens.They
are based on two soluble reactants that come together to makeone insoluble
product, the precipitate(Figure).
-Thesereactions depend on the formation of lattices (cross-links) when antigen and
antibody existin optimal proportions.[ it isknown aszoneofequivalence andappears
to us asprecipitation].
-Excessof either component reduceslattice formation andsubsequentprecipitation.](https://image.slidesharecdn.com/electrophoresis-ppt-240318115911-4e3a7e8f/75/ELECTROPHORESIS-and-its-types-ppt-pptx-77-2048.jpg)


















