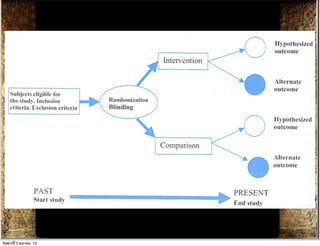
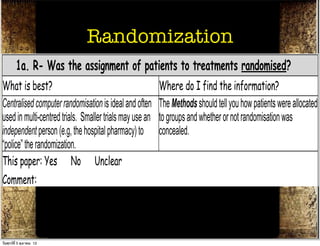
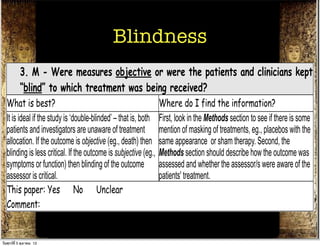
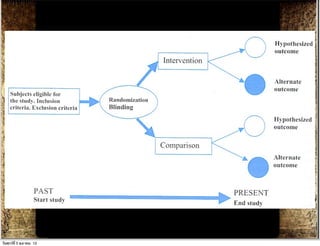
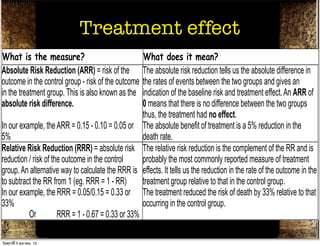
The document discusses how to critically appraise medical journal articles on therapy. It explains that the critical appraisal involves assessing whether the results of a trial are valid by evaluating factors like randomization, blinding, and follow-up of patients. It also involves determining the size of the treatment effect based on metrics like relative risk and absolute risk reduction, and assessing how precisely the treatment effect is estimated. Finally, the critical appraisal considers how to apply the trial results to patient care.