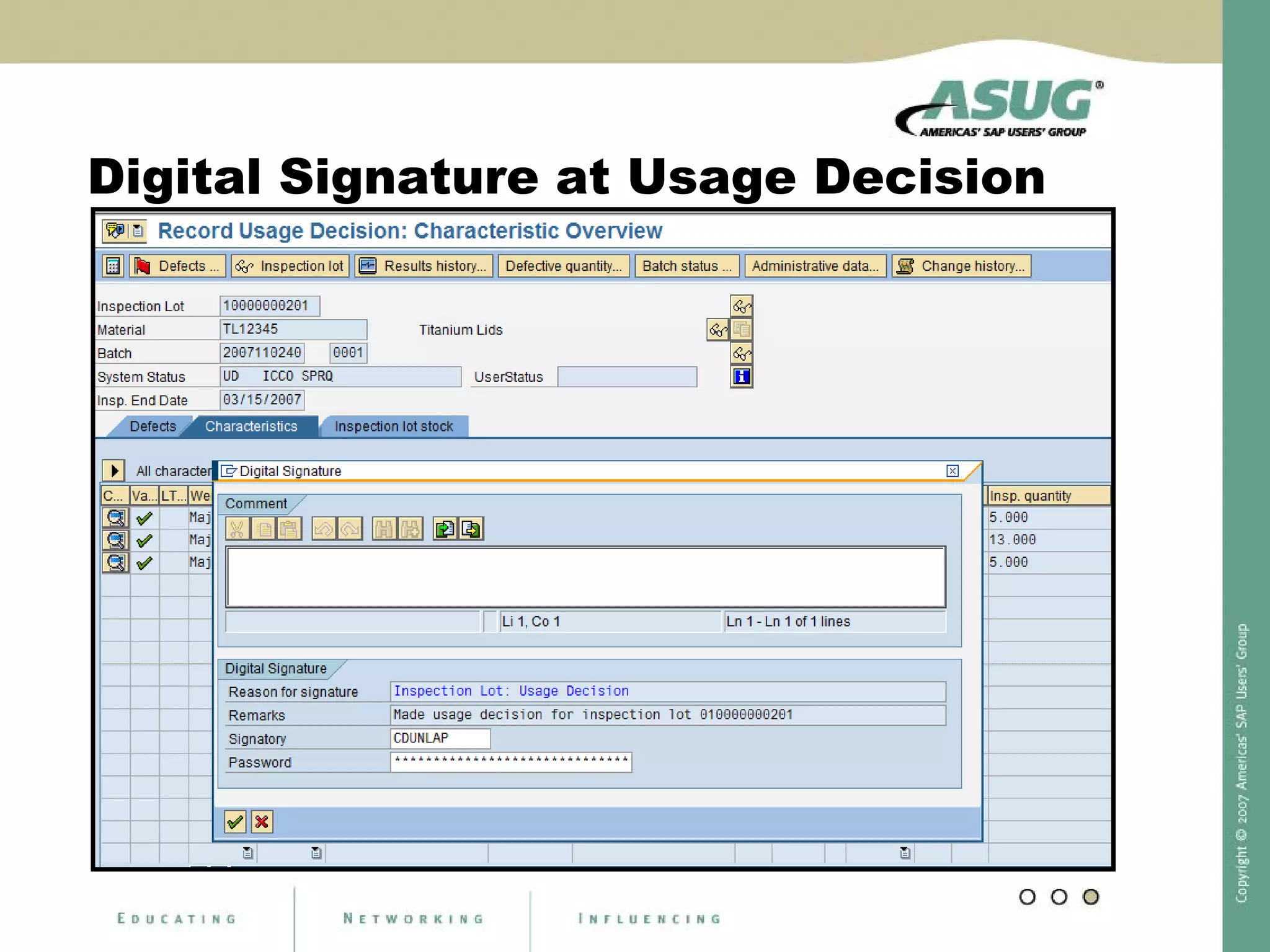
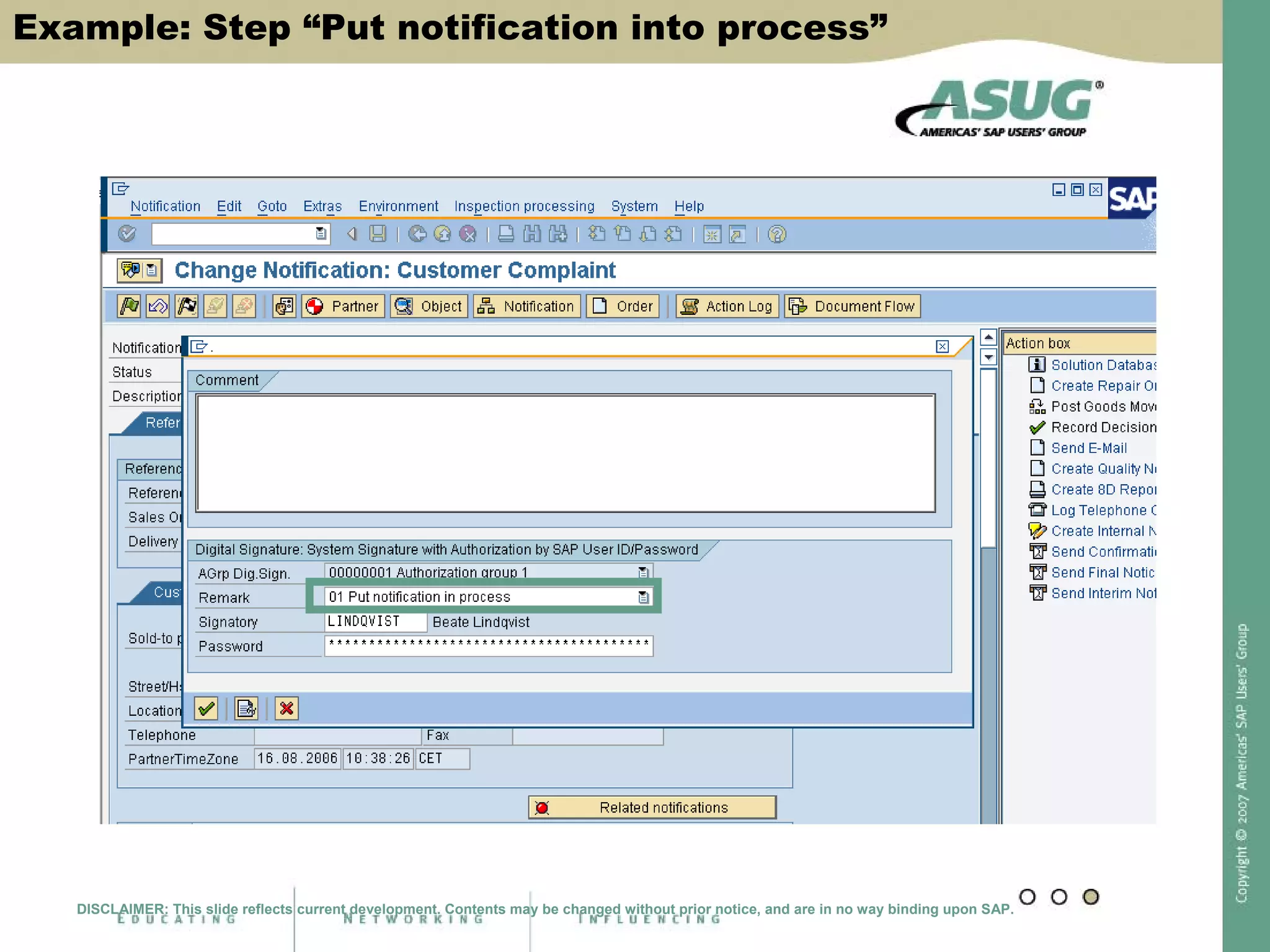
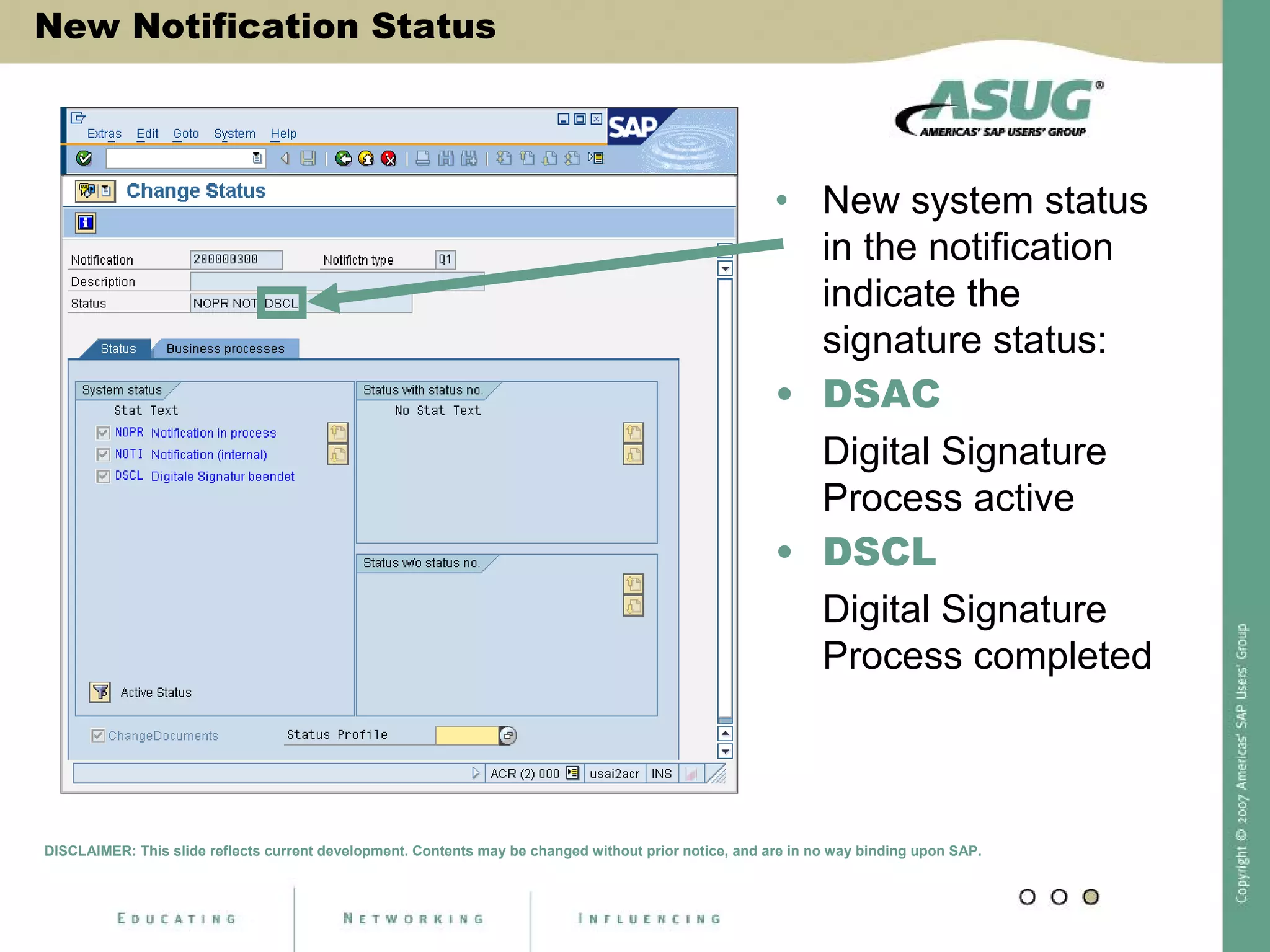
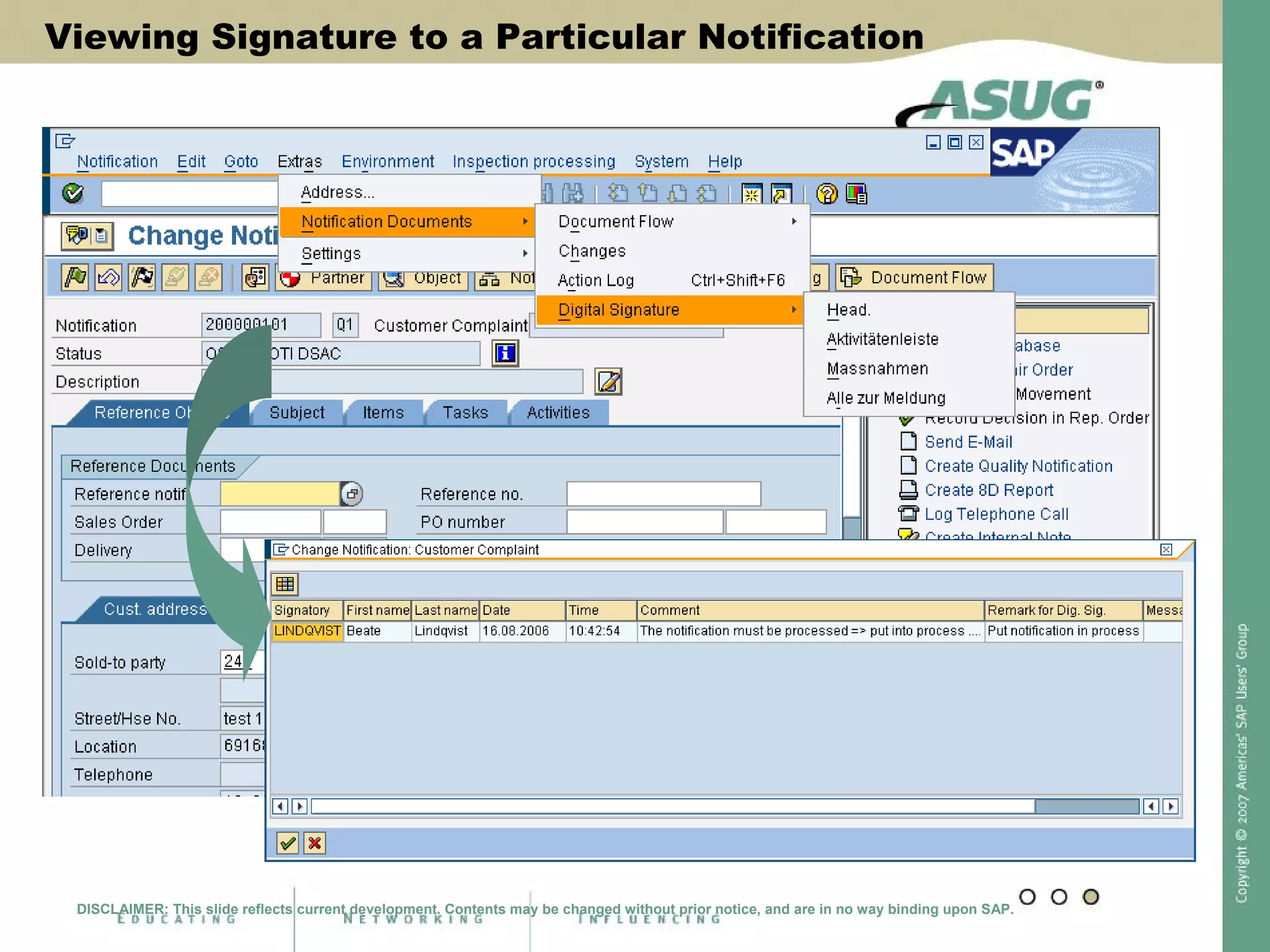
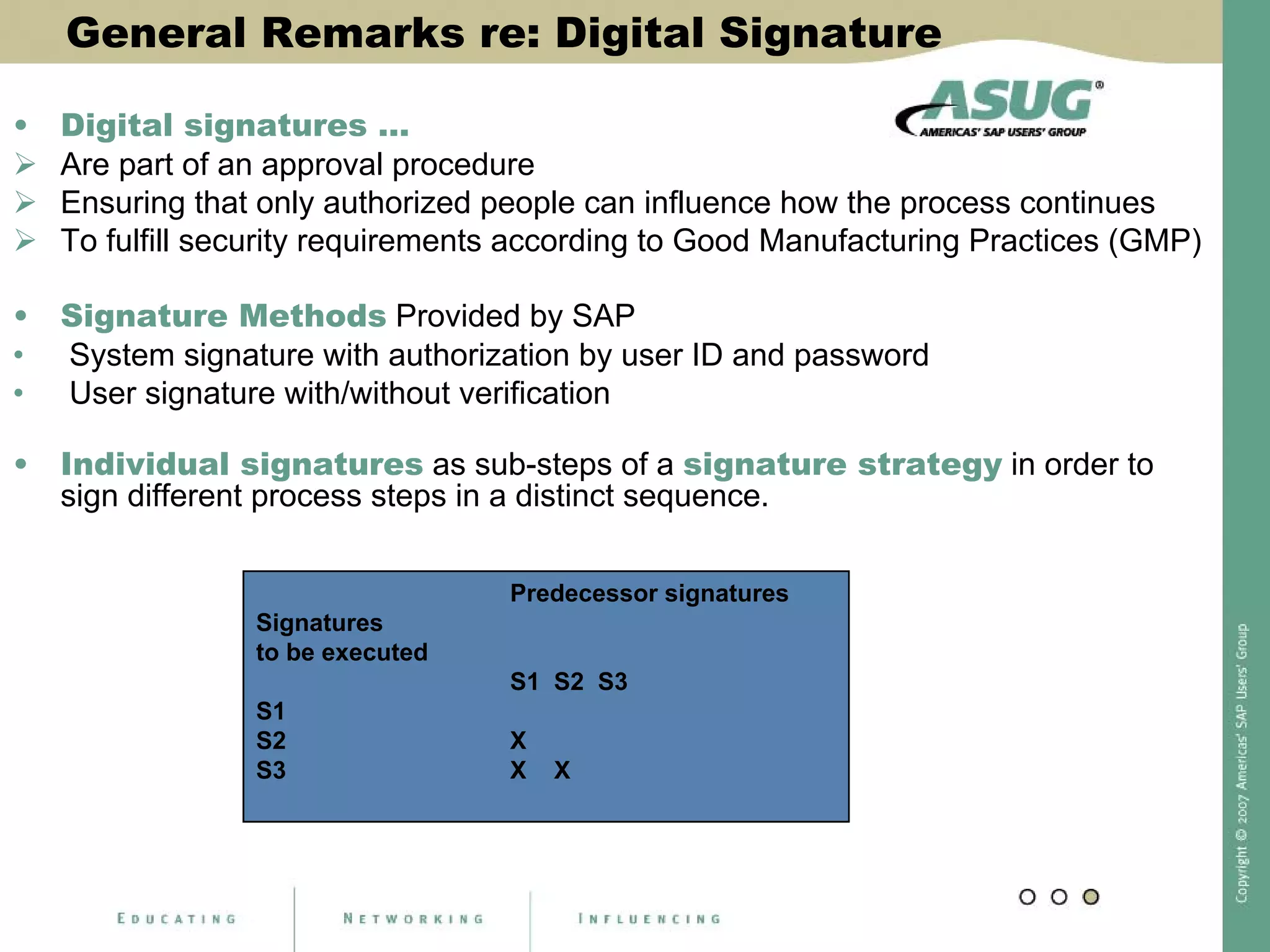
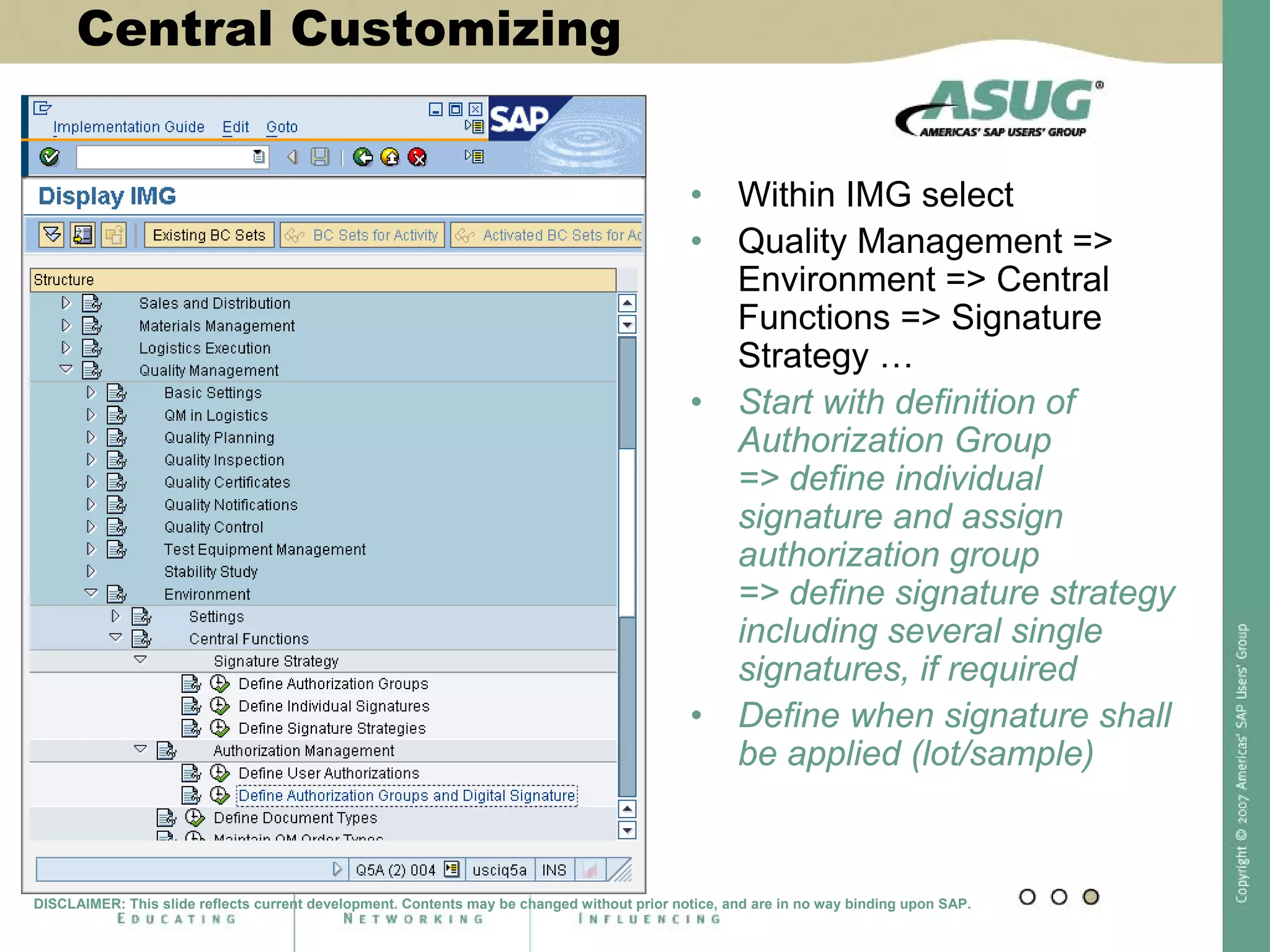
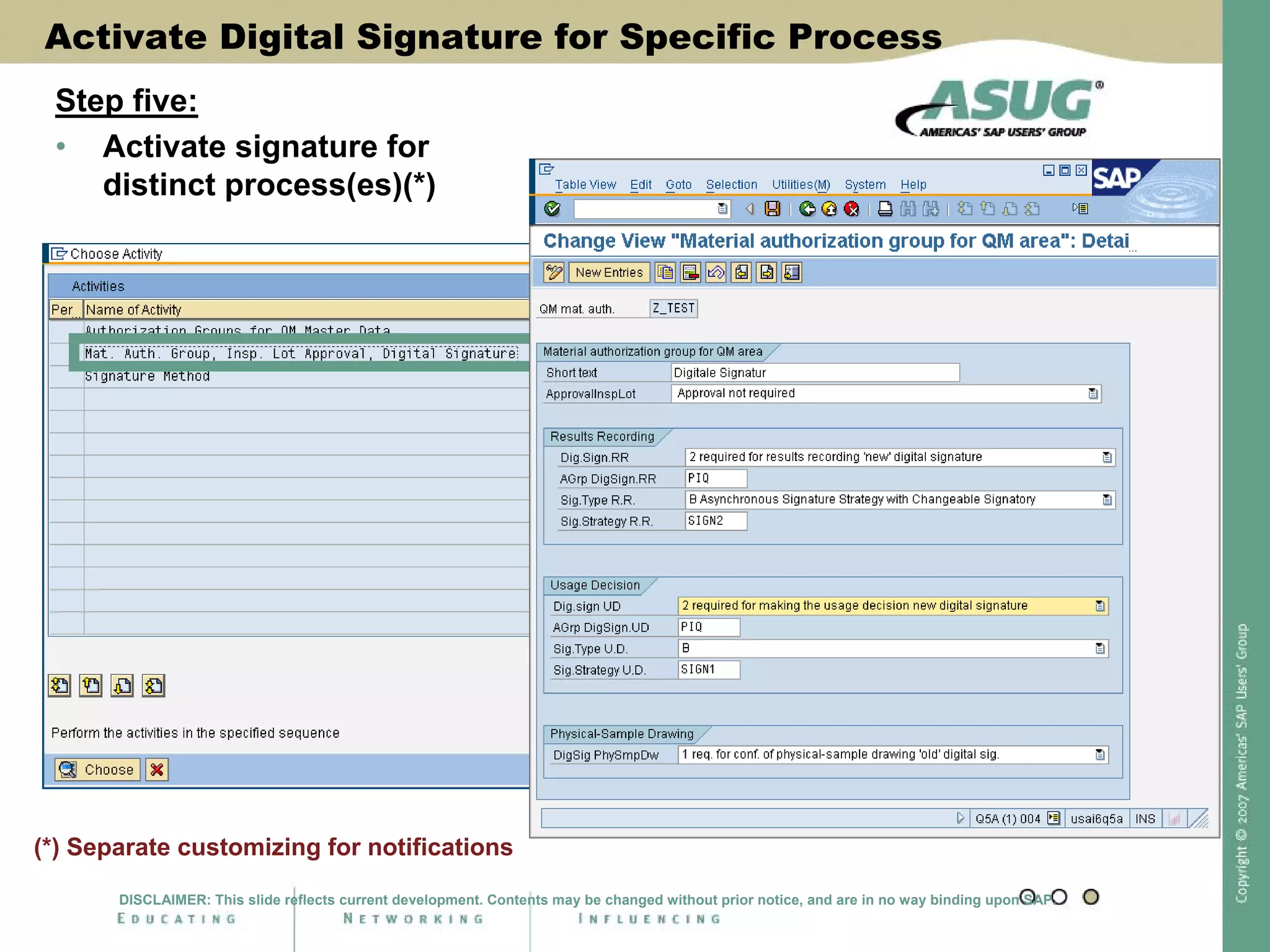
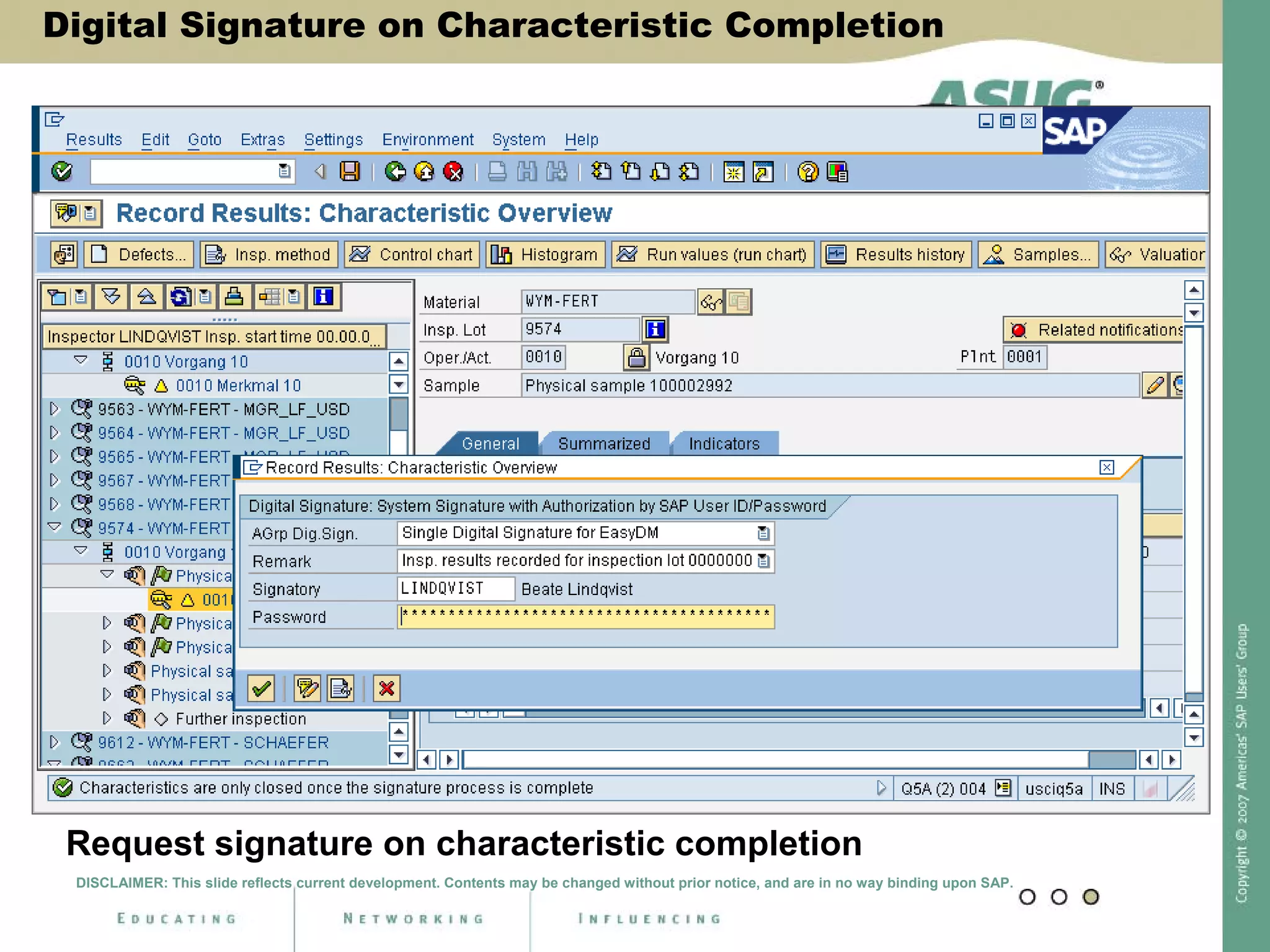
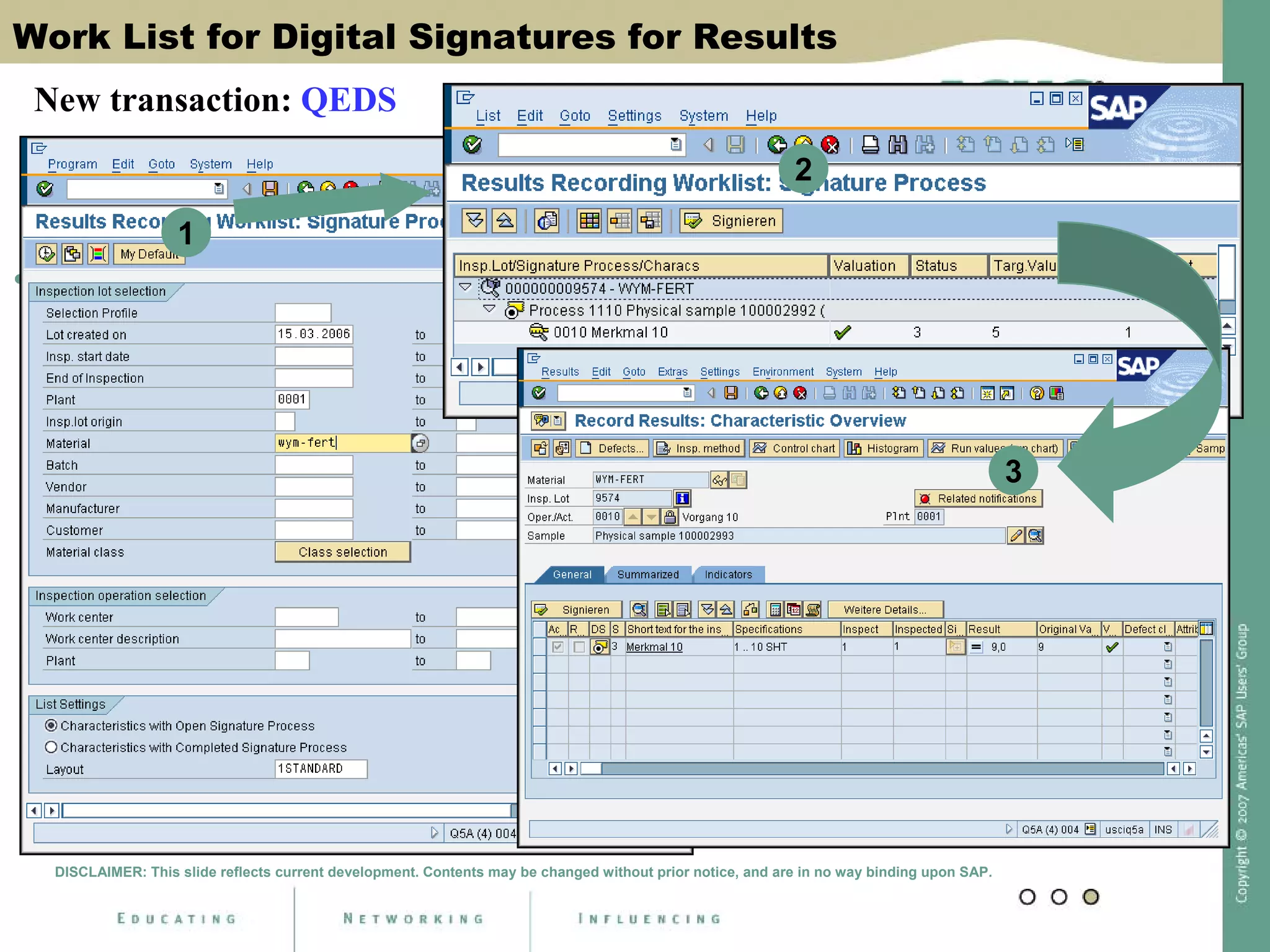
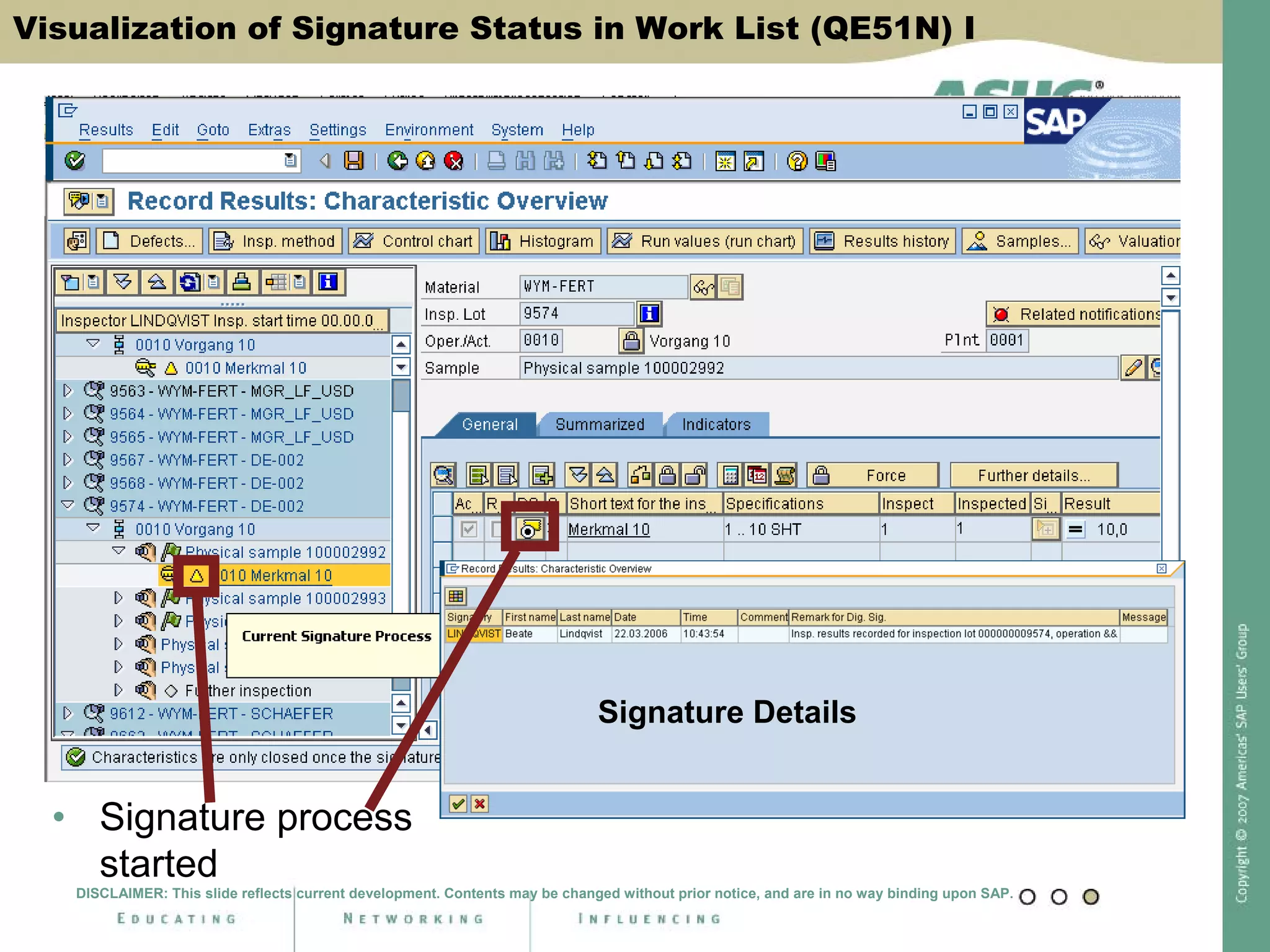
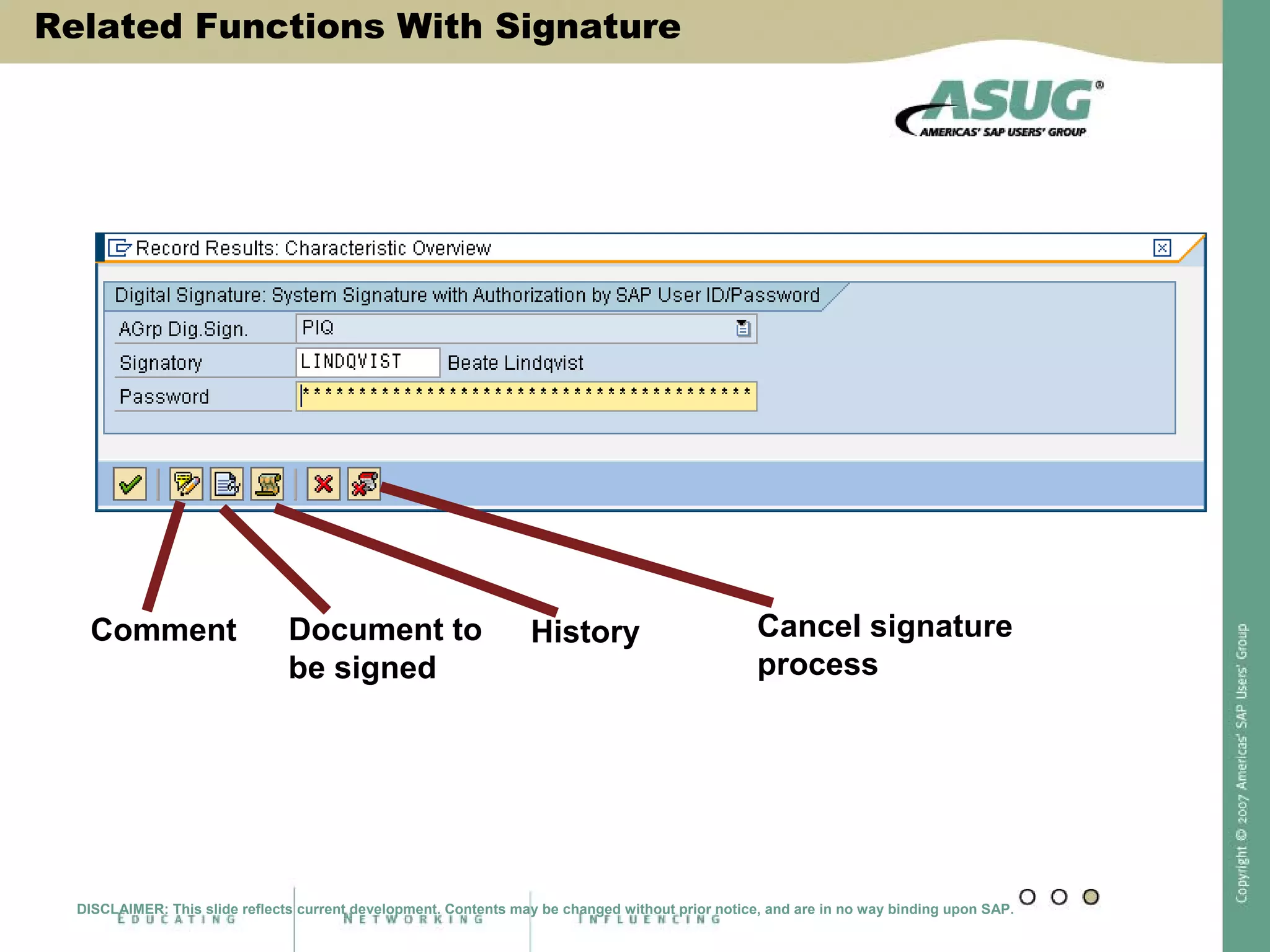
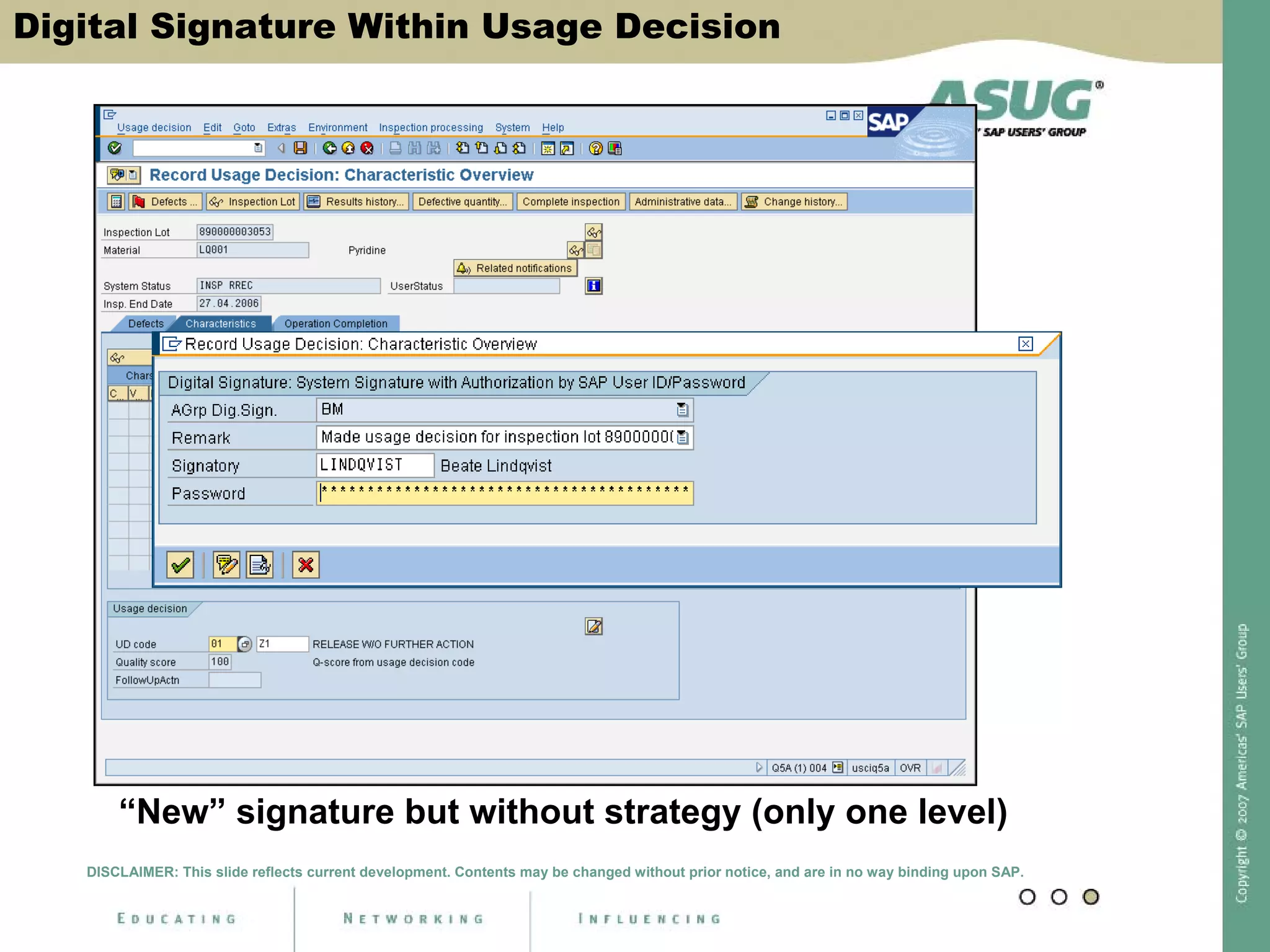
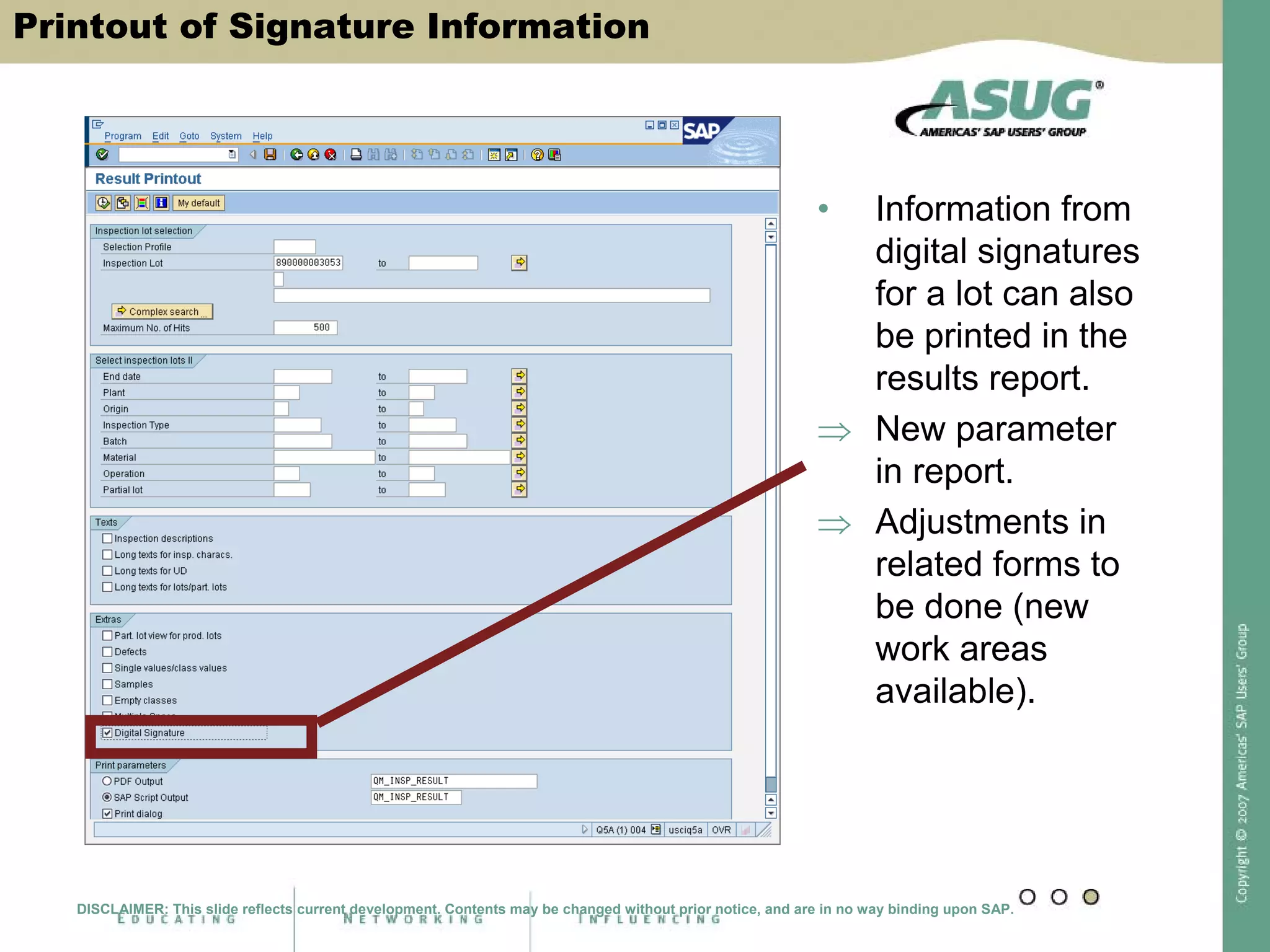
Digital signatures can be used in SAP QM to meet regulatory requirements like the FDA's Part 11 regulation. SAP software uses digital signatures to ensure electronic records and signatures are trustworthy, reliable, and compatible with regulatory work. New functionality in SAP ERP 2005 will provide additional controls over digital signatures, including signature strategies, sequences, and settings for mandatory comments. Digital signatures can be applied to quality notifications, inspection lots, and physical samples to document approval of processes and ensure only authorized users can progress workflows. Customizing is used to define authorization groups, individual signatures, signature strategies, and activate digital signatures for specific quality processes.