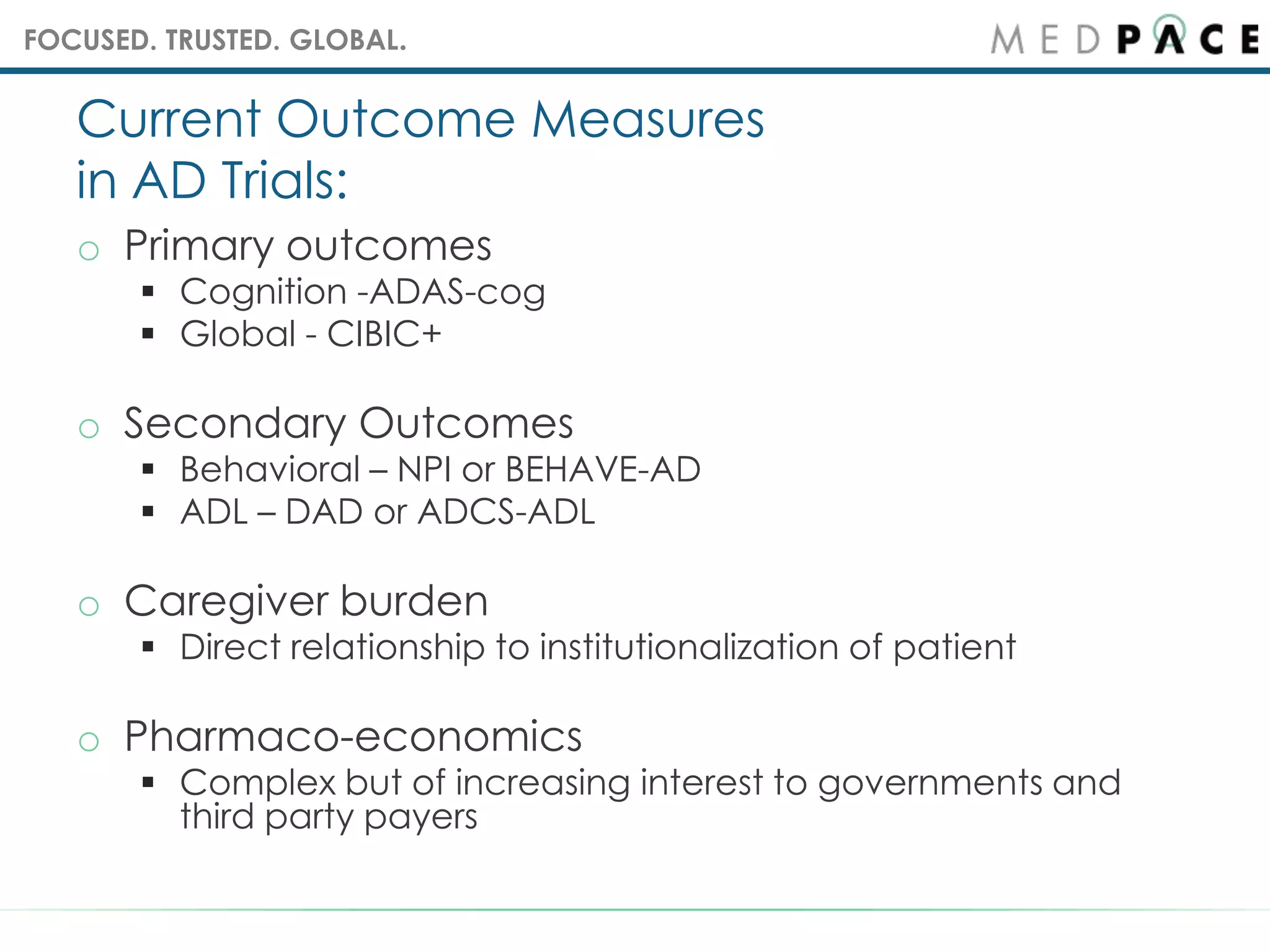
The document outlines the complexities of Alzheimer's disease (AD), including its pathology, clinical presentation, and the current lack of effective disease-modifying therapies. It emphasizes the importance of identifying therapeutic targets and the necessity for biomarkers to improve trial outcomes. Key treatment approaches discussed include symptomatic therapies and cognitive enhancement, while also highlighting the challenges faced in clinical trials and the evaluation of treatment efficacy.