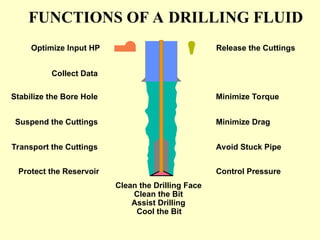
The document discusses drilling fluids as engineered tools that enhance drilling performance, minimize costs, and reduce environmental impacts. It outlines various components and functions of water-based and oil-based drilling fluids, emphasizing their chemical makeup, properties, and significance in drilling operations. New developments in drilling fluid technology, including thermally activated mud emulsions and formate-based drill-in fluids, are also mentioned, highlighting their advantages and specific applications.


![Water-based drilling fluid chemicals
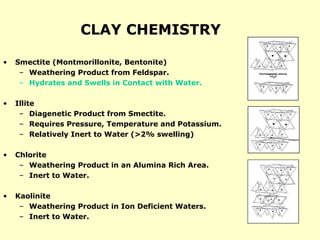
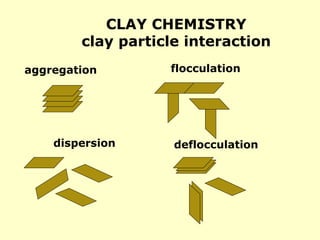
• Lime = Ca(OH)2 – pH buffer in case of H2S
• Ironite Sponge = Fe3O4 – H2S scavenger
• Zinc carbonate = ZnCO3 – H2S scavenger
• [Sulphonated] asphalt = plugging agent for shale formations
• Walnut shells, mica flakes, marble chips = lost circulation material
•Calcium carbonate = CaCO3 – 1) acid-soluble weighting material –
2) Lost circulation material](https://image.slidesharecdn.com/4-240903095630-8df4d35e/85/drilling-fluids-at-drilling-rigs-and-cement-5-320.jpg)
![H2O H+
+ OH-
pH = -log [H+
] where H+
is the hydrogen concentration in mol/l
pOH = -log [OH-
] where OH-
is the hydroxide concentration in mol/l
pH + pOH = 14
pH range
Example:
pH = 10 pOH = 4 [OH-
] concentration = 10-4
mol/l (1 mol = 17 gram)
Caustic soda added to water: NaOH Na+
+ OH-
Lime added to water: Ca(OH)2 Ca2+
+ 2OH-
(largely insoluble)
<7 = Acidic
7
>7 = Alkaline](https://image.slidesharecdn.com/4-240903095630-8df4d35e/85/drilling-fluids-at-drilling-rigs-and-cement-6-320.jpg)