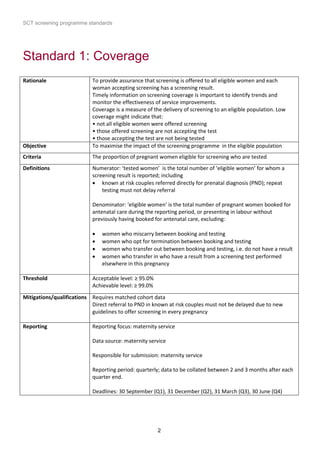
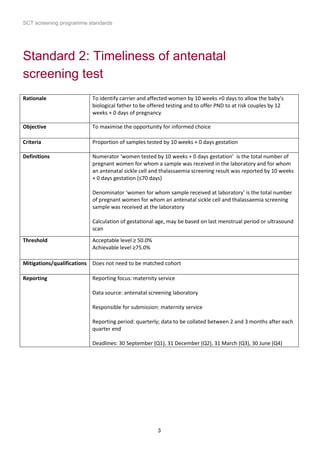
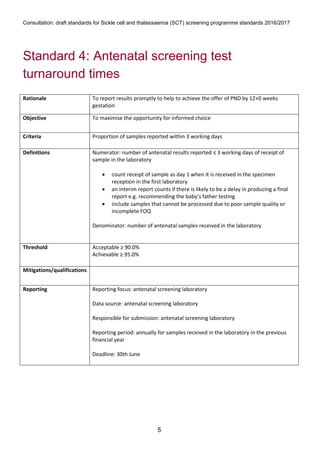
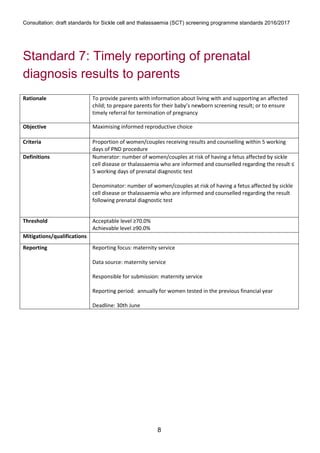
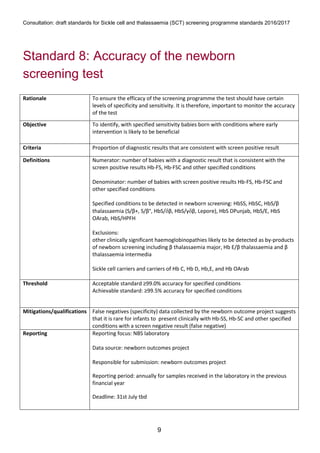
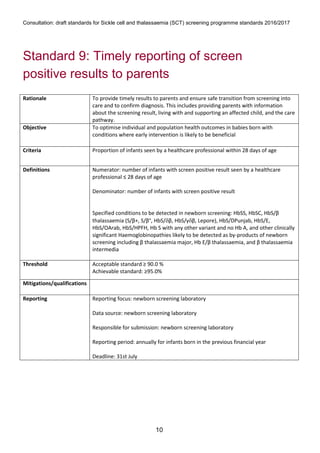
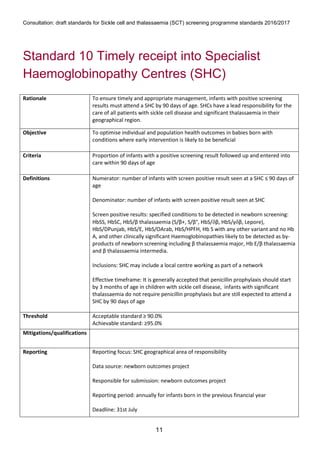
1) The document outlines standards for a sickle cell and thalassemia (SCT) screening program, including standards related to coverage, timeliness of screening tests, completion of questionnaires, turnaround times, counseling and offering of prenatal diagnosis, and timeliness of prenatal diagnosis.
2) The standards provide definitions, calculations, thresholds, and reporting requirements for each measure.
3) The objectives are to maximize screening and diagnosis opportunities to allow informed reproductive choices.