The document discusses the dissolution process of ionic compounds in water and the use of energy cycle diagrams to calculate related thermochemical quantities. It provides several examples of constructing energy cycle diagrams to calculate lattice energies and enthalpies of hydration for ionic compounds such as NaCl, LiCl, KF, and CaCl2. It also presents sample questions and solutions relating to these topics.
![DISSOLUTION PROCESS OF IONIC PRODUCT
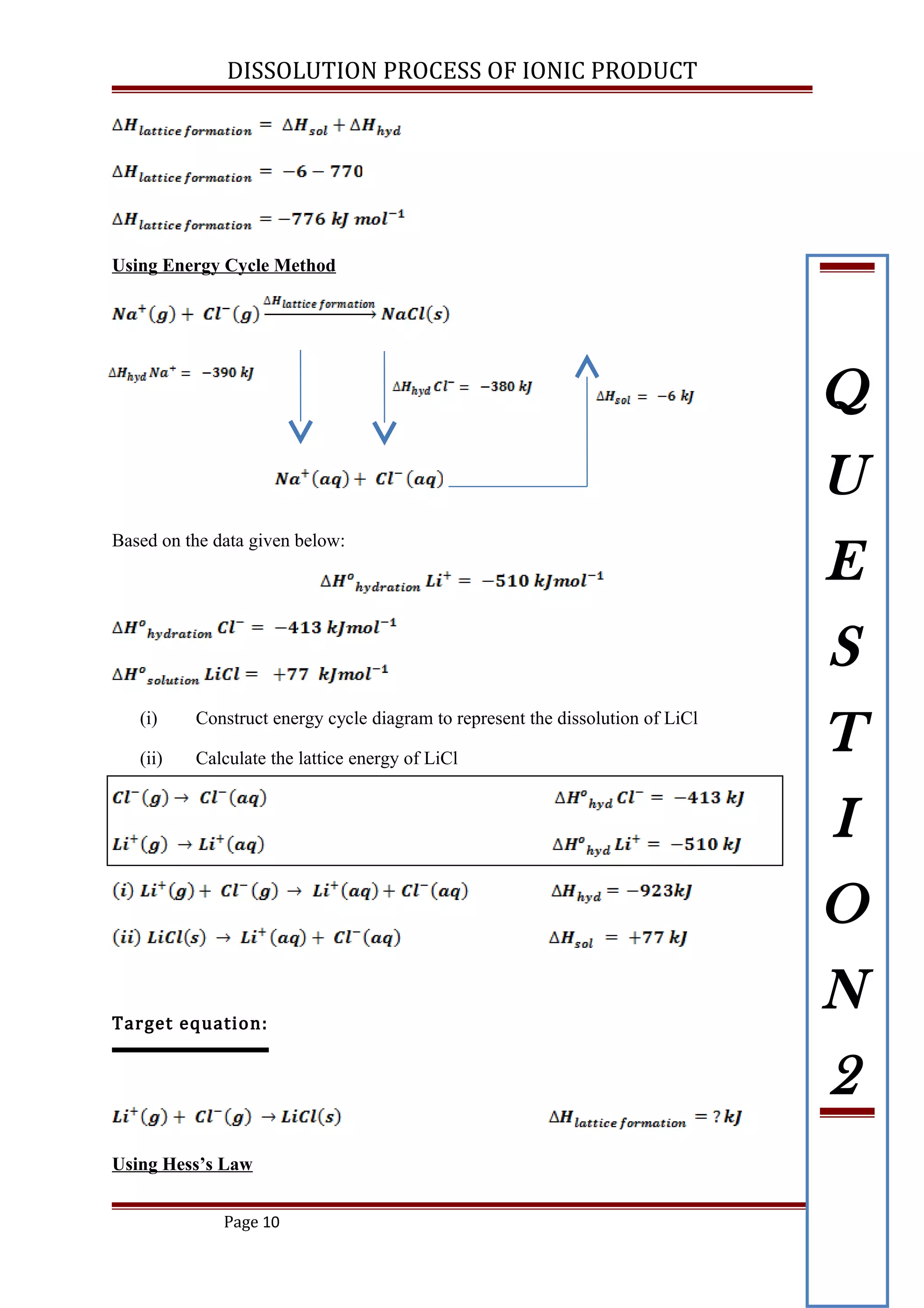
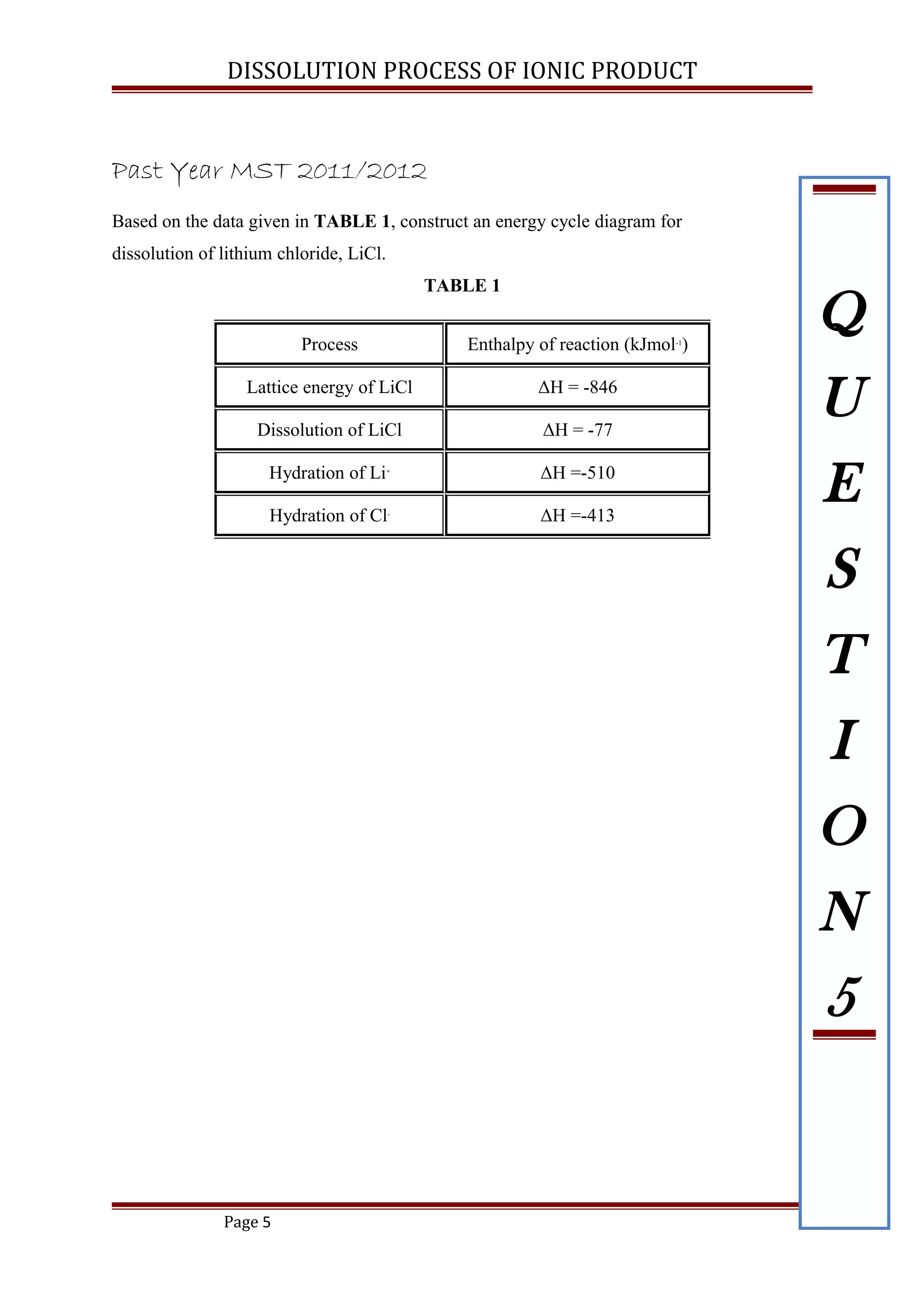
Based on the data given below:
(i) Construct energy cycle diagram to represent the dissolution of NaCl
(ii) Calculate the lattice energy of NaCl
[ ANS: -776 kJ mol-1
]
Page 1
Q
U
E
S
T
I
O
N
1](https://image.slidesharecdn.com/dissolutionprocessofionicsolid-160221173111/75/Dissolution-process-of-ionic-solid-1-2048.jpg)
![DISSOLUTION PROCESS OF IONIC PRODUCT
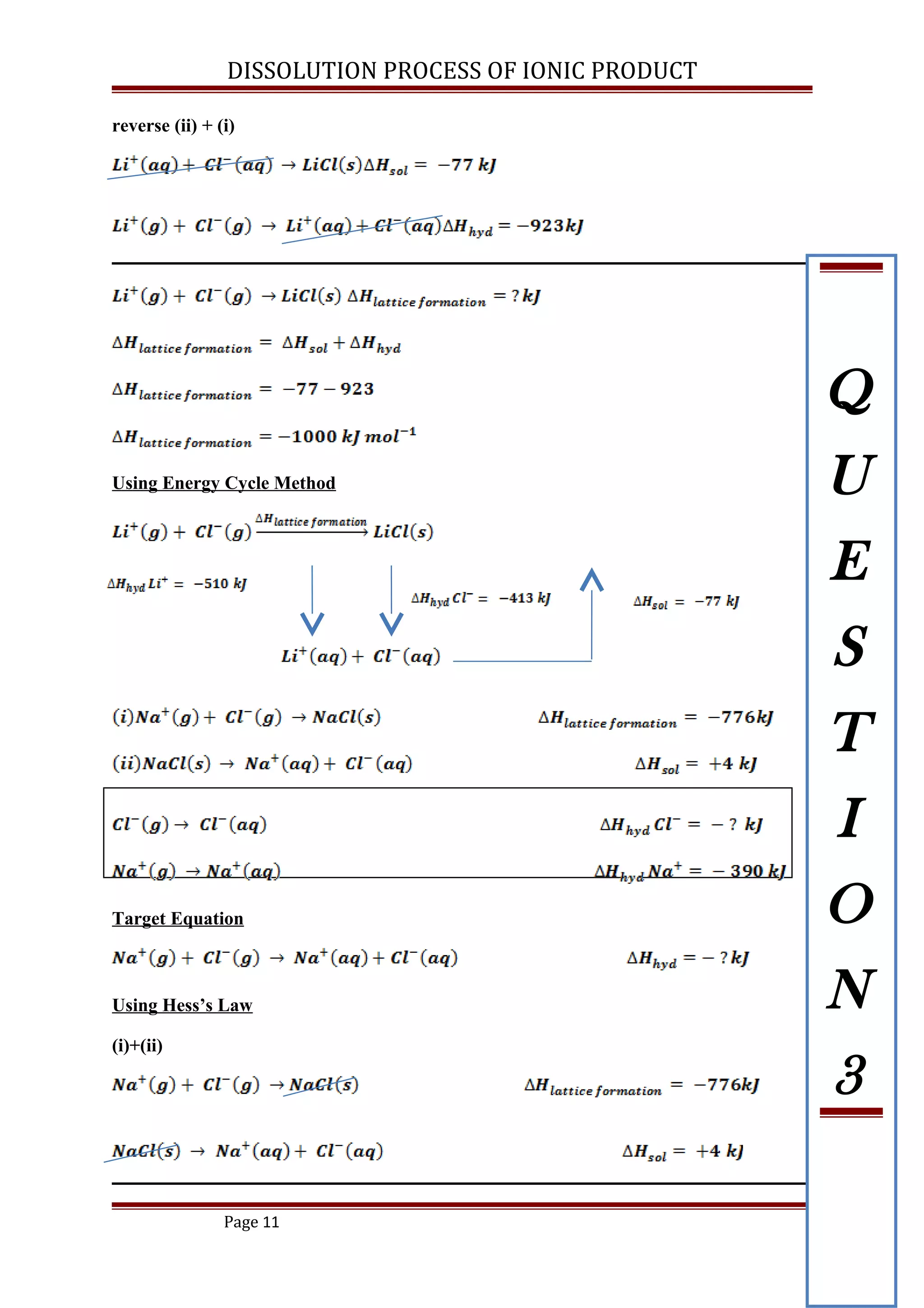
Based on the data given below:
(i) Construct energy cycle diagram to represent the dissolution of LiCl
(ii) Calculate the lattice energy of LiCl
[ ANS: -1000 kJ mol-1
]
Page 2
Q
U
E
S
T
I
O
N
2](https://image.slidesharecdn.com/dissolutionprocessofionicsolid-160221173111/75/Dissolution-process-of-ionic-solid-2-2048.jpg)
![DISSOLUTION PROCESS OF IONIC PRODUCT
Tutorial Question 18: Page 12 ( THERMOCHEMISTRY)
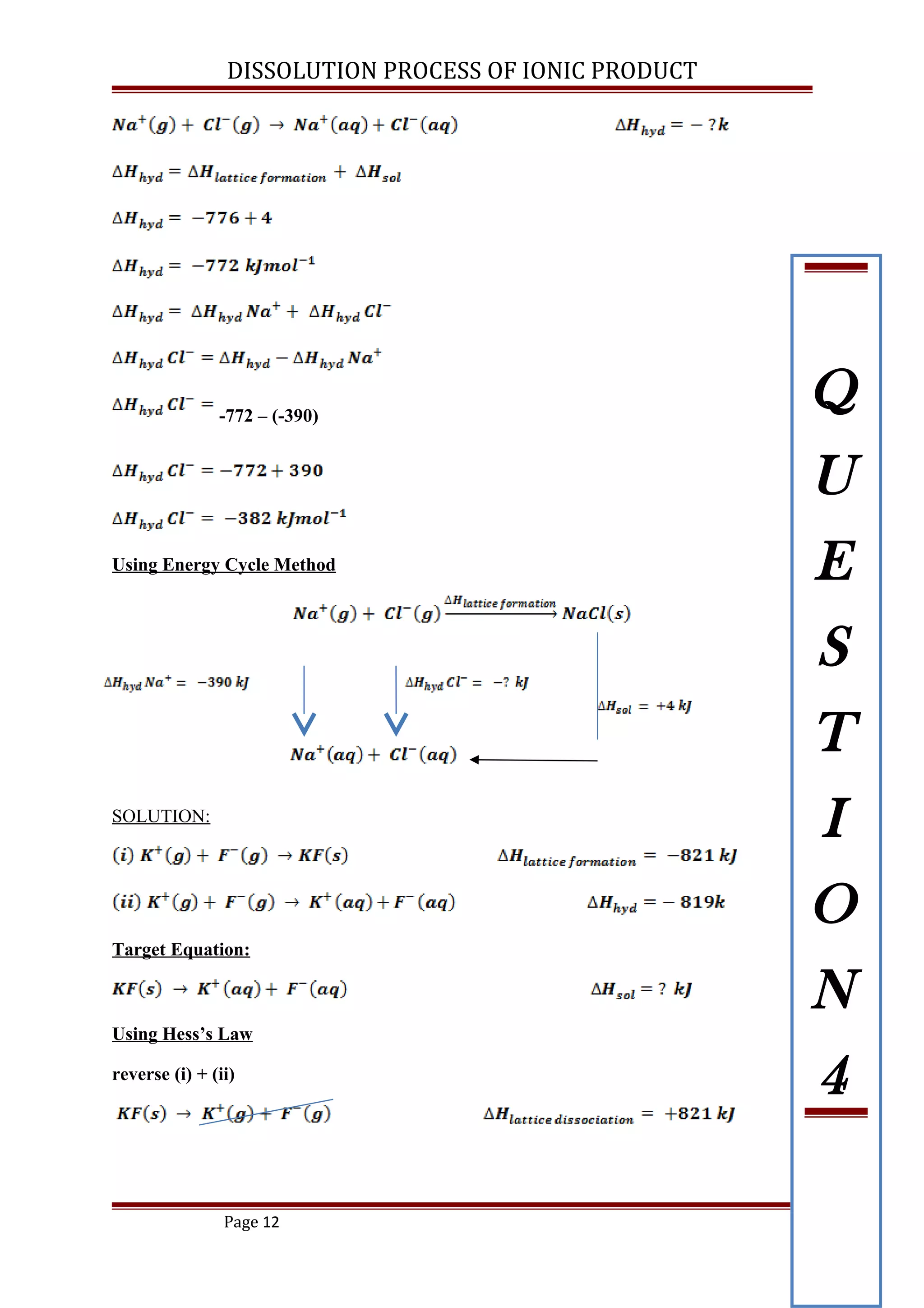
The lattice energy of NaCl is -776 kJ mol-1
and the enthalpy change when 1 mole
of solid NaCl dissolved in water is +4 kJ mol-1
. If the enthalpy of hydration of Na+
is 390 kJ mol-1
, calculate the enthalpy of hydration of Cl-
in the dissolution process
of NaCl using the energy cycle. [ ANS: -382 kJ mol-1
]
Page 3
Q
U
E
S
T
I
O
N
3](https://image.slidesharecdn.com/dissolutionprocessofionicsolid-160221173111/75/Dissolution-process-of-ionic-solid-3-2048.jpg)
![DISSOLUTION PROCESS OF IONIC PRODUCT
Tutorial Question 19: Page 12 ( THERMOCHEMISTRY)
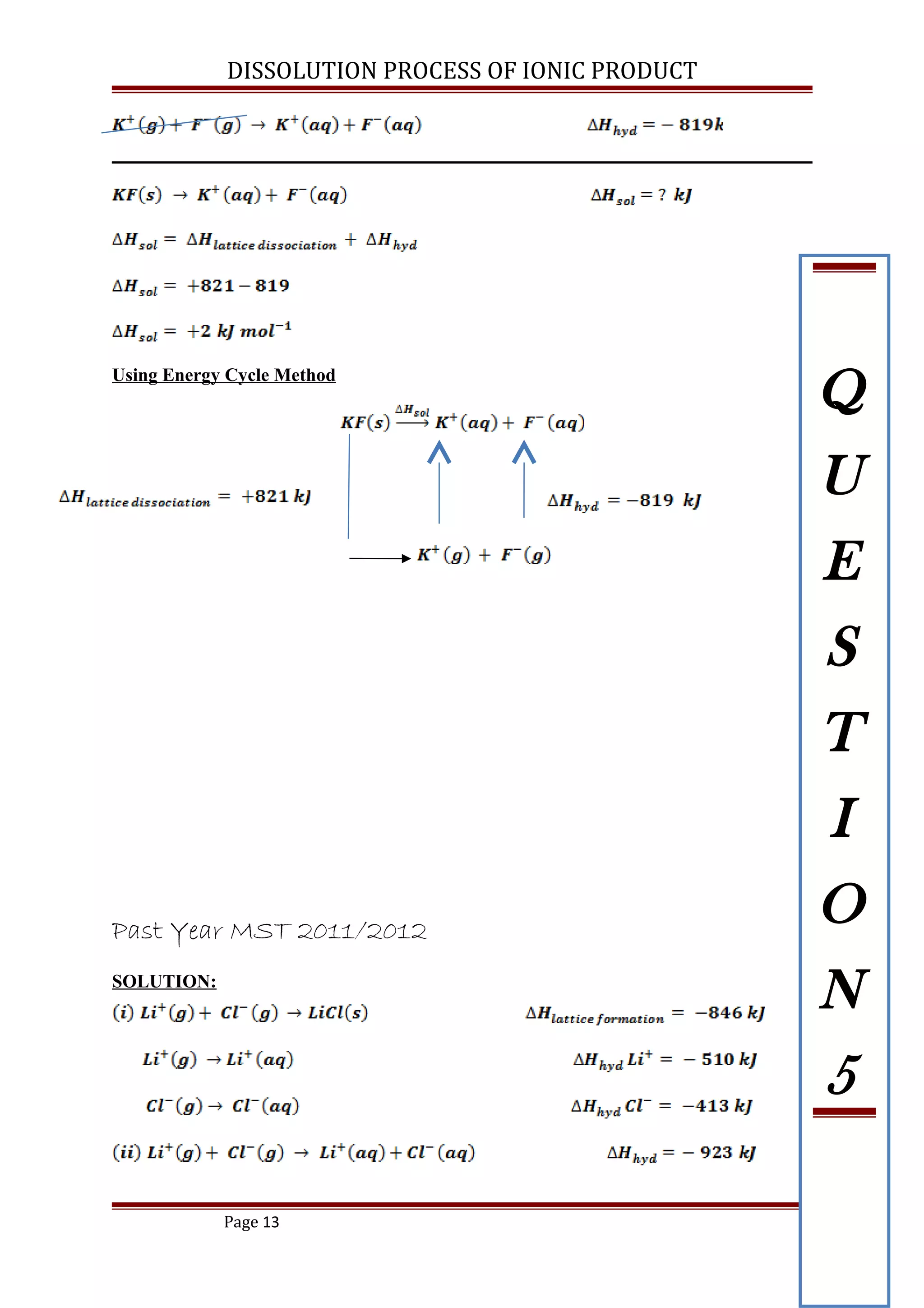
The lattice energy for potassium fluoride is -821 kJ mol-1
and its enthalpy of
hydration is -819 kJmol-1
. Calculate enthalpy of solution for potassium fluoride.
[ ANS: +2 kJ mol-1
]
Page 4
Q
U
E
S
T
I
O
N
4](https://image.slidesharecdn.com/dissolutionprocessofionicsolid-160221173111/75/Dissolution-process-of-ionic-solid-4-2048.jpg)
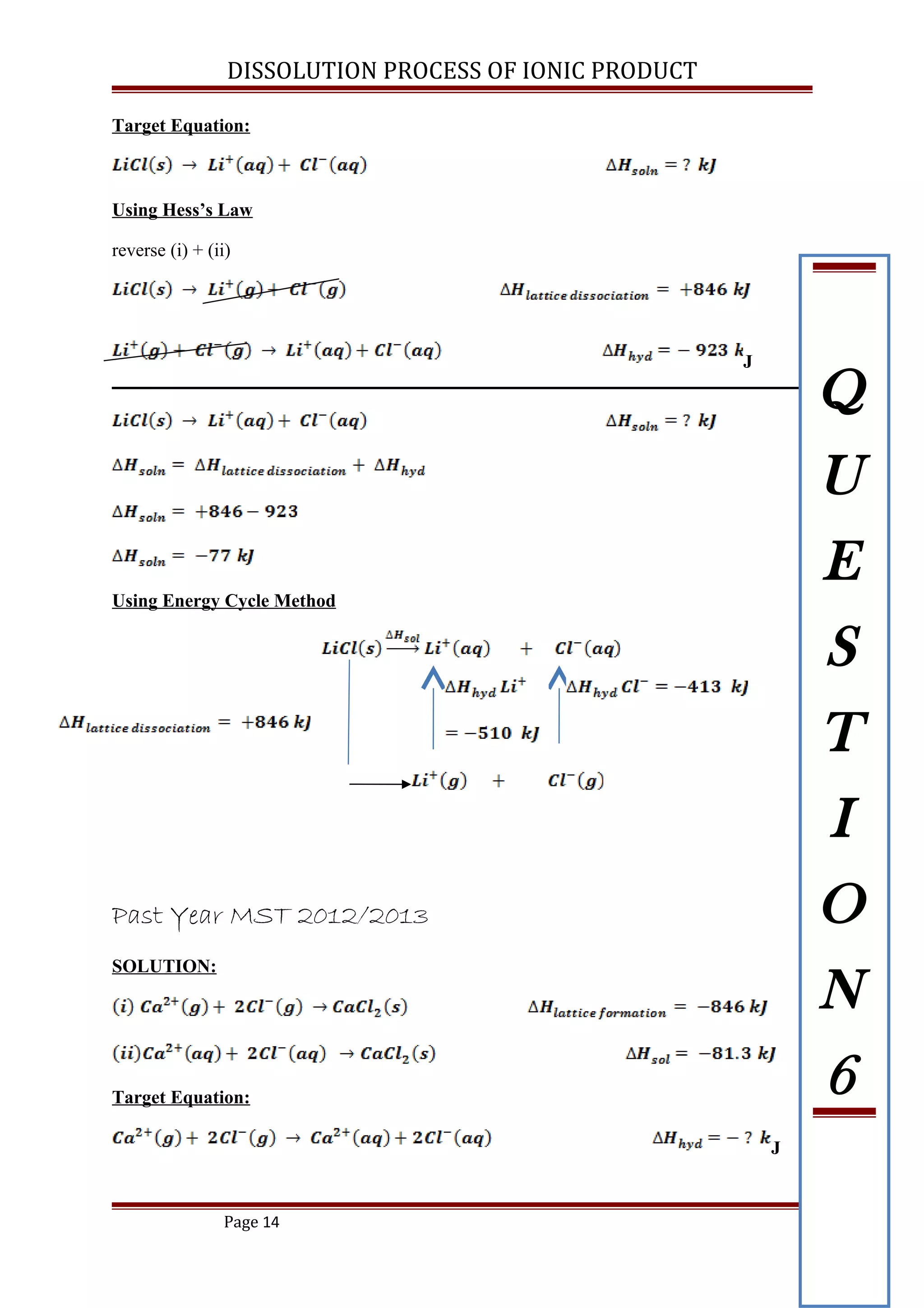
![DISSOLUTION PROCESS OF IONIC PRODUCT
Past Year MST 2012/2013
The enthalpy of solution for calcium chloride crystal is -81.3 kJ mol-1
. The lattice energy of
CaCl2 is -2249 kJ mol-1
. Using an energy cycle method, calculate the enthalpy change for
the reaction below:
[ANS: ]
Page 6
Q
U
E
S
T
I
O
N
6](https://image.slidesharecdn.com/dissolutionprocessofionicsolid-160221173111/75/Dissolution-process-of-ionic-solid-6-2048.jpg)
![DISSOLUTION PROCESS OF IONIC PRODUCT
Past Year PSPM 2 2012/2013
Calculate the enthalpy of hydration of Cl-
in the dissolution process of LiCl in water using
energy cycle method.
Given:
Lattice energy of LiCl = -846 kJ mol-1
Enthalpy of solution of LiCl in water = -37 kJ mol-1
Enthalpy of hydration of Li+ = -510 kJ mol-1
[ANS: ]
Page 7
Q
U
E
S
T
I
O
N
7](https://image.slidesharecdn.com/dissolutionprocessofionicsolid-160221173111/75/Dissolution-process-of-ionic-solid-7-2048.jpg)
![DISSOLUTION PROCESS OF IONIC PRODUCT
Given :
(a) Name each of these three enthalpy changes.
(b) Suggest a reason why enthalpy of hydration are exothermic processes?
(c) Write an equation, including state symbols, for the enthalpy change
of solution of NaF.
(d) Using energy cycle method, calculate the enthalpy change of solution of NaF.
[ ANS: +71 kJ mol-1
]
Page 8
Q
U
E
S
T
I
O
N
8](https://image.slidesharecdn.com/dissolutionprocessofionicsolid-160221173111/75/Dissolution-process-of-ionic-solid-8-2048.jpg)