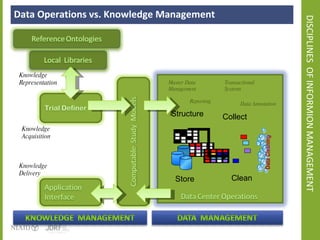
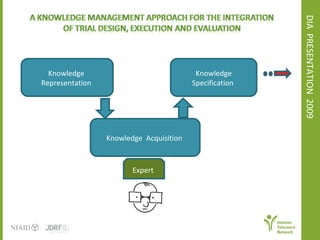
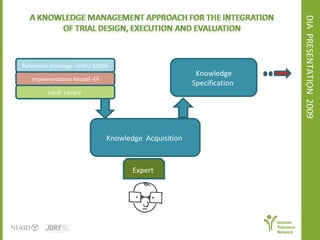
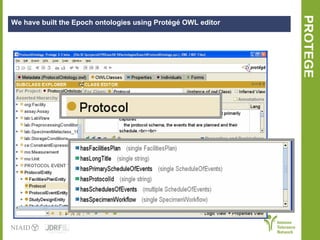
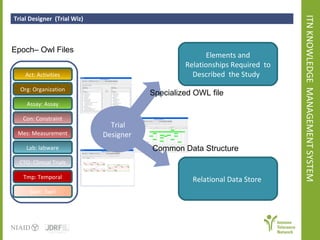
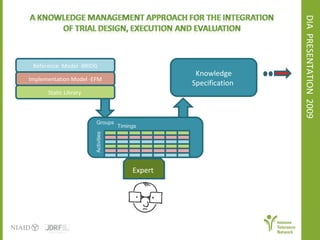
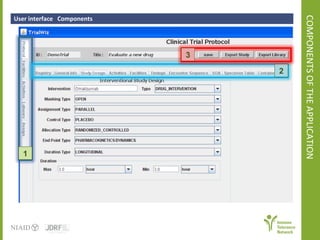
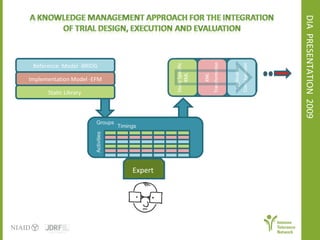
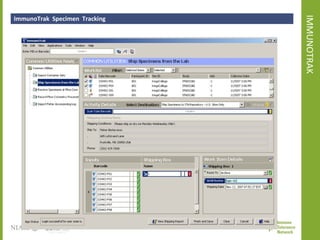
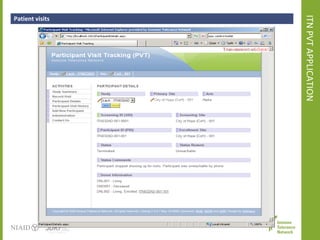
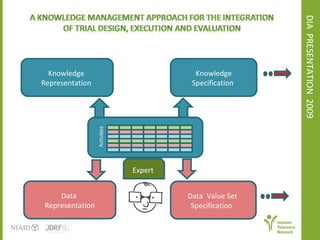
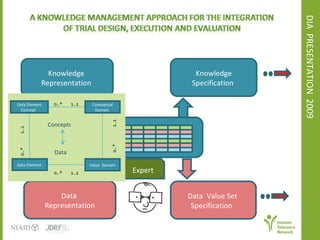
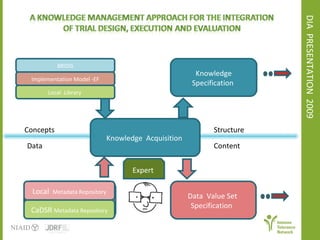
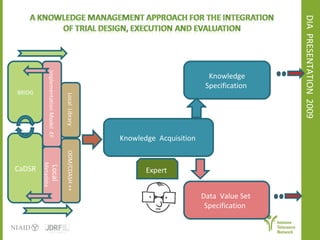
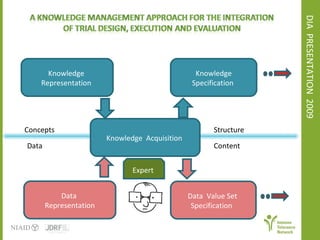
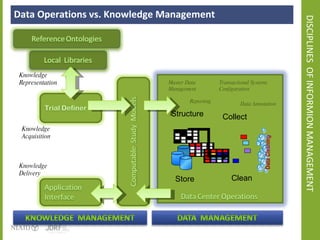
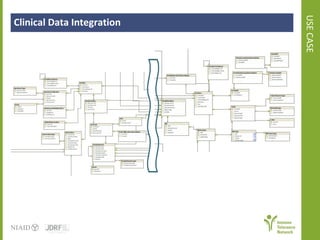
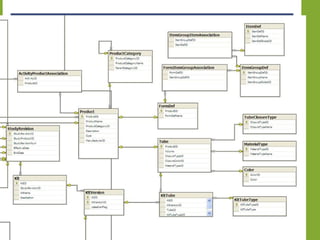
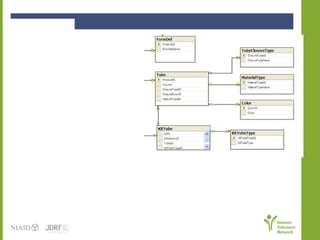
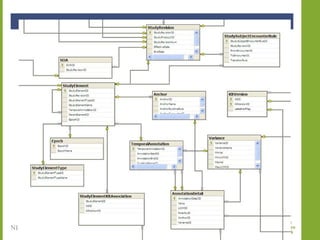
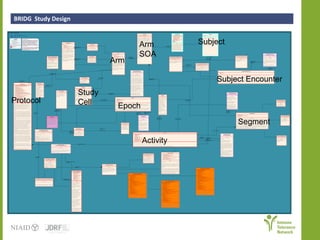
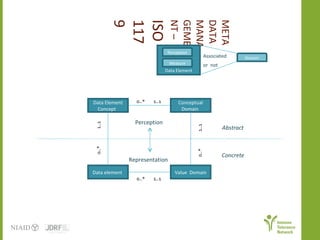
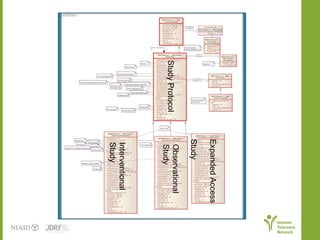
The document discusses knowledge management systems used in clinical trials at the Immune Tolerance Network (ITN). It describes the ITN's mission to advance immune tolerance therapies through high-quality clinical trials integrated with mechanistic research. It outlines some of the key challenges around balancing adaptability and reusability and evolving technologies with data longevity. It also describes some of the key disciplines and components of ITN's knowledge management system including knowledge representation, acquisition, and delivery as well as transactional systems, data annotation, and reporting.