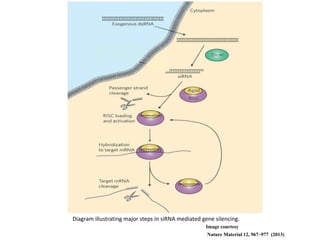
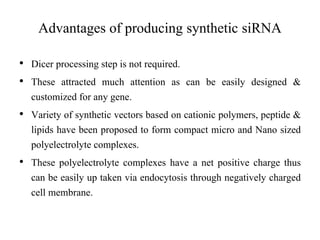
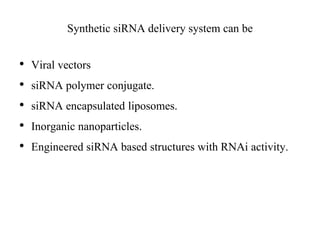
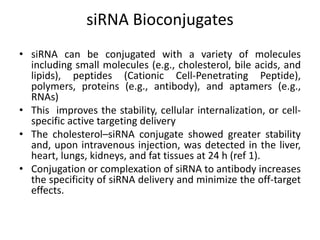
1. Delivery systems of siRNA include synthetic vectors based on cationic polymers, peptides, and lipids that form polyelectrolyte complexes with siRNA for cellular uptake.
2. These complexes have a positive charge allowing for endocytosis through the negatively charged cell membrane.
3. Effective delivery systems overcome challenges of poor cellular uptake and rapid enzymatic degradation, with nanotechnology offering targeted platforms.

![Cationic liposomes
• Many cationic liposome/lipid-based systems can be
selected such as:
– lipofectamine2000,
– DOTAP (N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N trimethyl
ammonium methyl-sulfate) and
– DOTMA (N-(1-(2,3-dioleyloxy)propyl)-N,N,N-trimethyl
ammonium chloride).
• Lipofectamine 2000 is a cationic liposome based
reagent that provides high transfection efficiency and
high level of transgene expression in a range of
mammalian cell types in vitro.](https://image.slidesharecdn.com/deliverysystems-170614112321/85/Delivery-systems-7-320.jpg)